Enhancing Gravimetric Precision Under Extreme Conditions: The Role of the Quadrupole Magnetic Control System (QMS) in the AMI MSB
Download PDF
Introduction
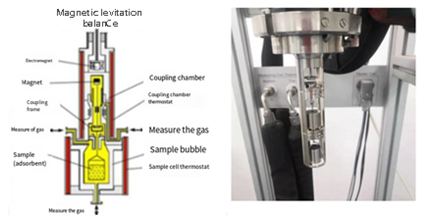
Magnetic Suspension Balances (MSBs) have become indispensable tools for gravimetric analysis under extreme conditions—including high pressures, elevated temperatures, and corrosive atmospheres—where traditional microbalances fail. A critical innovation in AMI’s next-generation MSB is the Quadrupole Magnetic Control System (QMS), which advances the precision and stability of mass measurements even further.
This application note explains the unique advantages of the QMS, how it addresses the challenges of high-pressure adsorption experiments, and how it improves the reliability and flexibility of the AMI MSB platform.
Technical Challenge: Precision Under Dynamic Forces
In high-pressure sorption studies, maintaining stable suspension of the sample while loading or when subjected to external disturbances is a key challenge. Traditional MSB designs can experience:
- Transient forces during sample loadingcausing reading instabilities.
- Influence from external magnetic fieldsaffecting the electromagnetic coupling system, leading to signal drift or noise over long experiments.
Such disturbances can compromise the accuracy of both static sorption isotherms and kinetic adsorption curves—particularly in dynamic or long-duration experiments.
QMS Solution: Locking Stability and Shielding Precision
The Quadrupole Magnetic Control System (QMS) resolves these challenges through two core innovations:
- Suspension Locking Mechanism
During sample loading, the QMS activates a quadrupole magnetic fieldthat locks the suspension mechanism in place. This eliminates transient forces that could otherwise displace the sample or distort early data points. - External Magnetic Field Shielding
The QMS generates a magnetic field precisely tuned to counteract external magnetic interferences. By stabilizing the electromagnetic coupling system, the QMS ensures that even in laboratories with variable magnetic fields, the measurement remains accurate and repeatable.
Key Benefits for Sorption and Gravimetric Applications
- Measurement Stability Over Time:
QMS minimizes drift during long-term weighing experiments—critical for adsorption kinetics and endurance testing. - Improved Accuracy in Dynamic Loading:
Locking the suspension during sample introduction reduces error and improves reproducibility, especially for delicate samples or small mass changes. - Broader Experimental Versatility:
By reducing sensitivity to external magnetic fluctuations, QMS-equipped MSBs can operate in a wider range of laboratory environments without performance degradation.
Typical Use Cases
- High-pressure gas sorption (up to 700 bar)
- Multi-component competitive adsorption
- Gravimetric density measurements at extreme conditions
- Long-duration kinetic adsorption studies
- Corrosive environment testing (e.g., H₂S, SO₂)
Conclusion
The QMS exemplifies AMI’s commitment to pushing the boundaries of gravimetric measurement under real-world and extreme conditions. By enhancing suspension control and eliminating external magnetic influences, the QMS not only safeguards precision but also expands the experimental capabilities of the AMI MSB platform.
For laboratories demanding the highest standards in sorption science, material research, and gas storage studies, QMS delivers measurable improvements in accuracy, repeatability, and operational confidence.
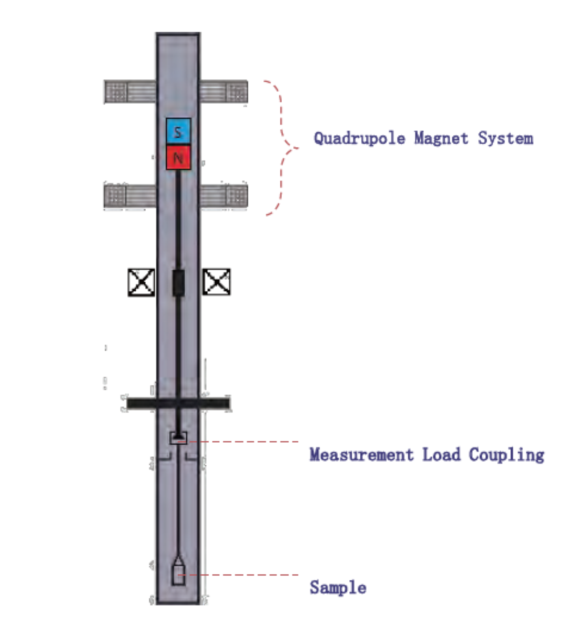
QMS-MSB Schematic
| 560 mT Field Distance | QMS Measurement Deviation | Measurement Deviation |
|---|---|---|
| 100 mm | ±2 μg | ±10 μg |
| 300 mm | ±1 μg | ±3 μg |
| 1000 mm | ±1 μg | ±1 μg |
QMS Performance: Measurement Deviation vs. External Magnetic Field Distance

 Products
Products
 Products
Products
 TEL: +1 262-877-3600
TEL: +1 262-877-3600
 EMAIL:sales@ami-instruments.com
EMAIL:sales@ami-instruments.com