Advanced Measurement Instruments
Copyright © 2025 Advanced Measurement Instruments
Carbon dioxide (CO₂) is a major greenhouse gas, and technologies for its capture and storage are central to combating climate change. Among the available approaches, adsorption using porous materials has gained significant traction due to its low energy demands and high efficiency. However, conventional CO₂ adsorption at ambient conditions suffers from low capacity and poor selectivity.
Supercritical CO₂ (scCO₂)—formed when CO₂ surpasses its critical point of 31.1°C and 7.39 MPa—exhibits a hybrid of gas and liquid properties: high diffusivity, tunable density, and low viscosity. These attributes make it uniquely useful in materials science, separation, and enhanced oil recovery (EOR).
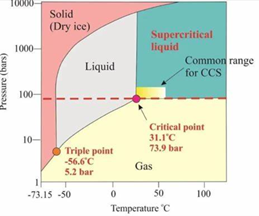
FIGURE 1 – Phase Diagram of Supercritical CO₂
Geological Sequestration
scCO₂ geological sequestration is a widely recognized method for long-term CO₂ storage. Injecting scCO₂ into deep geological formations—such as depleted reservoirs and saline aquifers—allows CO₂ to be trapped through structural, solubility, or residual mechanisms. Mineral carbonation reactions further convert CO₂ into stable carbonate solids, significantly reducing the risk of leakage [1].
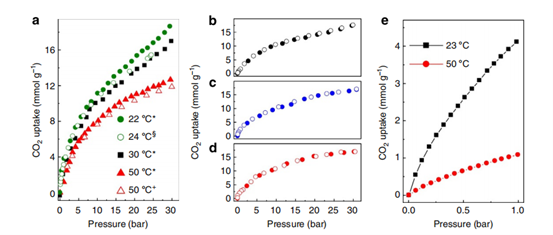
FIGURE 2– Investigation of CO₂ Adsorption under Normal vs. High Pressure
Enhanced Oil Recovery (CO₂-EOR)
EOR using scCO₂ serves both economic and environmental goals. When scCO₂ reaches miscibility pressure with crude oil (typically >10 MPa), it significantly reduces oil viscosity and improves flow characteristics.
EOR injection methods include:
scCO₂-EOR can increase recovery by 10–25% and extend oil field lifetimes by decades. In the U.S., such projects already sequester 30 million tons of CO₂ annually, or 1.5% of industrial emissions.
2.1 Why Magnetic Levitation?
Volumetric methods—though simple—require larger sample quantities and cannot effectively resolve kinetic behavior or low-pressure responses [2].
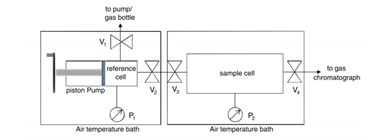
FIGURE 3 – Principle of Volumetric scCO₂ Testing
Gravimetric methods are preferred for high-precision adsorption work, but traditional balances suffer from:
AMI’s Magnetic Suspension Balance (MSB) overcomes these issues:
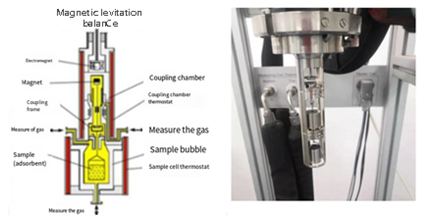
FIGURE 4 – Magnetic Levitation Instrument Structure
Case 1: Supercritical CO₂ Adsorption in Coal
High-pressure, high-temperature coal seams are ideal for CO₂ sequestration. Li and Ni et al. investigated the CO₂ adsorption behavior of Tunliu and Sihe coal under supercritical conditions [3].
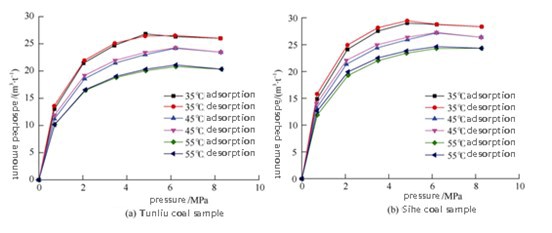
FIGURE 5 – Isothermal Adsorption/Desorption Curves of Two Coal Types
Key Observations:
However, corrections for excess vs. absolute adsorption and phase density assumptions are required to obtain true capacity data. Post-correction, adsorption shows stable increase with pressure and fully reversible desorption.
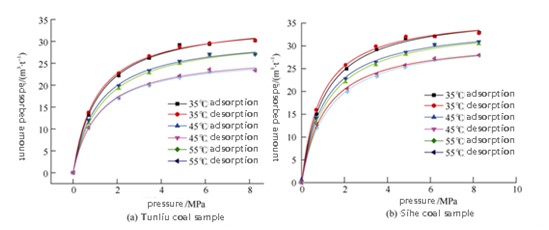
FIGURE 6– Density-Corrected Isotherms with Binary Langmuir Fitting
Case 2: Kinetics of scCO₂ Adsorption
Monitoring real-time uptake at fixed pressure provides kinetic insight. Adsorption is faster at higher pressure, but at supercritical pressures, adsorption approaches saturation more rapidly, reducing the number of available sites over time [5].
Studies by Charrière and Song show that:
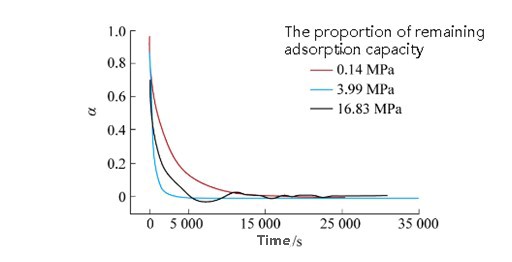
FIGURE 7– CO₂ Residual Adsorption vs. Time at Various Pressures
Case 3: CO₂-EOR in Shale Reservoirs
Due to the complexity of core testing in shale (tight pore networks), magnetic levitation offers a direct and non-invasive method to monitor oil extraction.
Li and Chen used MSB readings at fixed intervals and compared results to NMR, showing strong correlation [8].
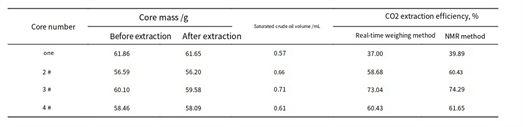
Table 1 – Comparison of Extraction Efficiency by Magnetic Levitation vs. NMR
With increasing CO₂ pressure:
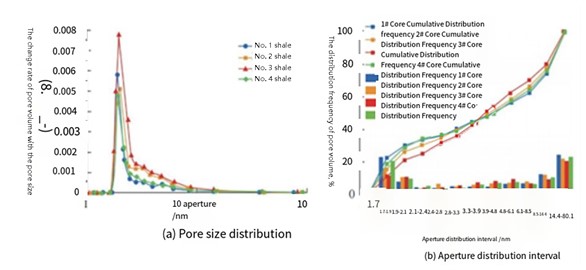
FIGURE 8– Pore Size Distribution of Tested Samples
4.1 Conditions
4.2 Results
BET analysis showed (on AMI Instruments):
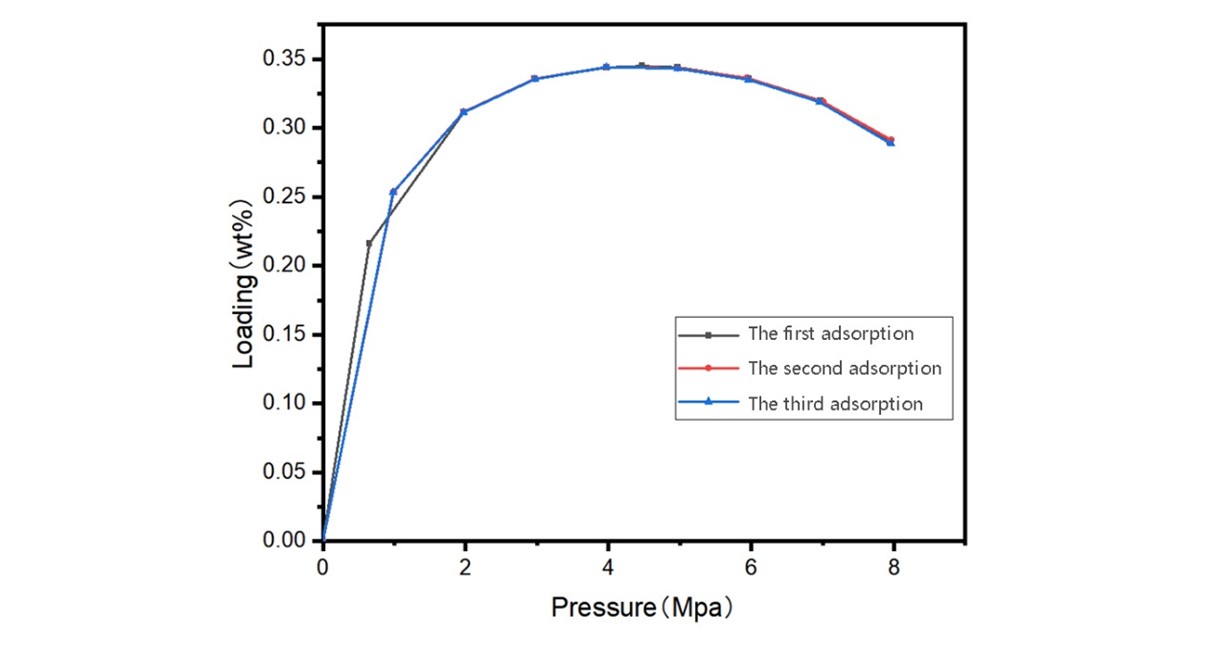
FIGURE 9 – Measured Supercritical CO₂ Adsorption Capacity
Magnetic suspension gravimetric systems are essential tools for modern supercritical CO₂ adsorption research. Their advantages include:
Looking forward, future research should address:
References
[1] Lv, Y., Tang, D., Xu, H., et al. CO₂ sequestration technology for improving coalbed methane recovery. Environmental Science and Technology, 2011, 34(5): 95–99.
[2] Sudibandriyo M., Pan Z., Fitzgerald J.E., et al. Langmuir, 2003, 19: 5323–5331.
[3] Li, Q., Ni, X., Wang, Y., et al. Coalfield Geology and Exploration, 2014, 42(3): 36–40.
[4] Fu, X., Qin, Y., Wei, C. Coalbed Methane Geology, China University of Mining and Technology Press, 2007.
[5] Charrière D., Pokryszka Z., Behra P. International Journal of Coal Geology, 2010, 81(4): 373–380.
[6] Song Y., Xing W., Zhang Y., et al. Adsorption, 2015, 21(1/2): 53–65.
[7] Siemons N., Wolf K.A.A., Bruining J. International Journal of Coal Geology, 2007, 72(3): 315–324.
[8] Li, B., Hou, J., Lei, Z., et al. Petroleum Drilling Technology, 2024, 52(4): 94–103.