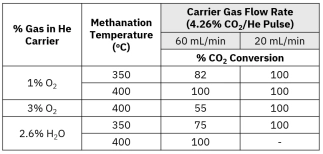
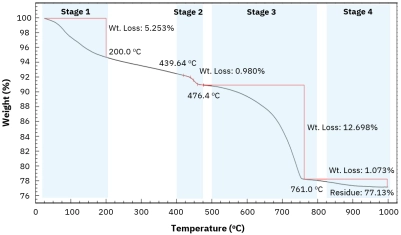
Understanding TPD kinetics, surface characterization, and desorption dynamics for heterogeneous catalyst analysis.[…]
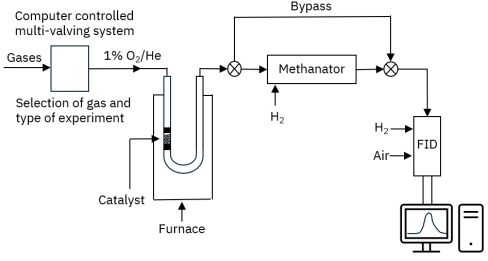
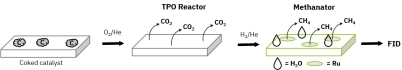
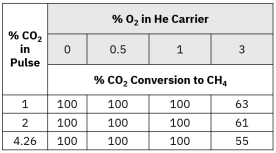
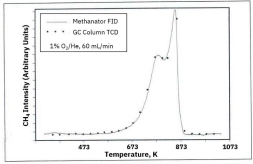
Methanation-enhanced FID detection offers significantly higher sensitivity and better signal precision compared to thermal conductivity detectors (TCD). It enables detection of carbon concentrations below 0.1%, provides real-time monitoring without requiring a GC column, and allows accurate differentiation of coke oxidation behavior—making it ideal for advanced catalyst research and industrial regeneration studies.