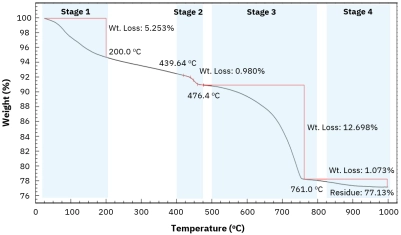
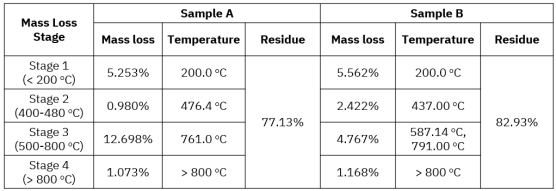
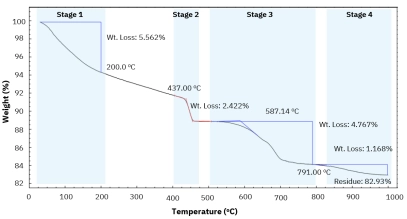
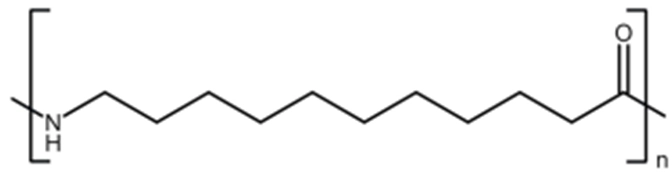
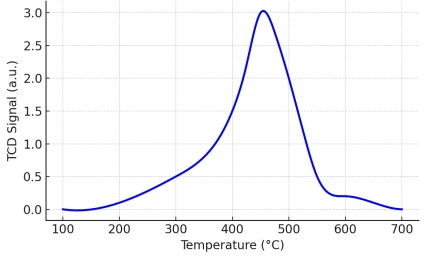
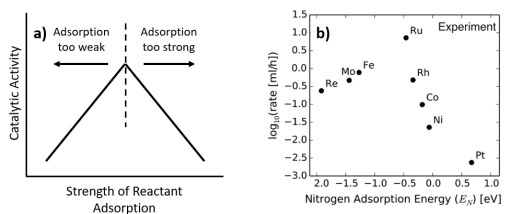
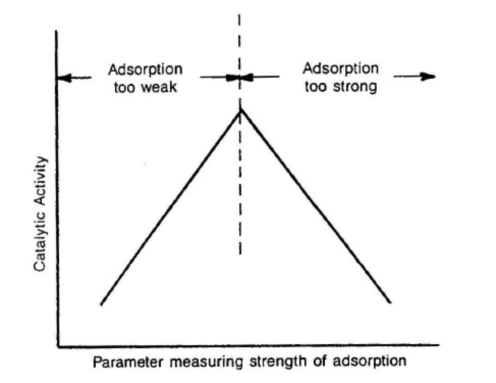
Polyamides (nylons) are widely used engineering thermoplastics because their molecular structure directly controls performance in real-world thermal environments.[…]
For reliable cement characterization, recommended TGA conditions include a heating rate of 10°C/min, a sample mass of approximately 20 mg, nitrogen atmosphere at 50 mL/min, and use of inert platinum crucibles. These parameters ensure accurate phase separation, minimal peak overlap, and reproducible quantification of hydration and carbonation products.