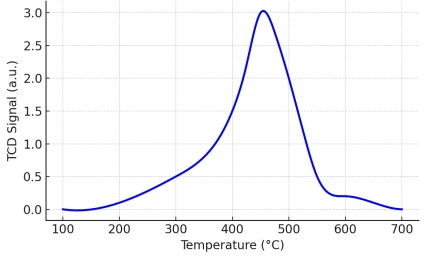
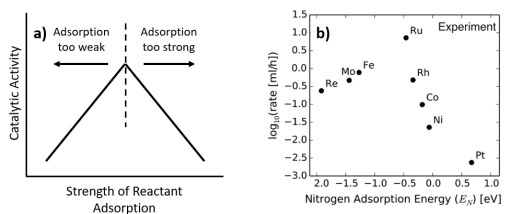
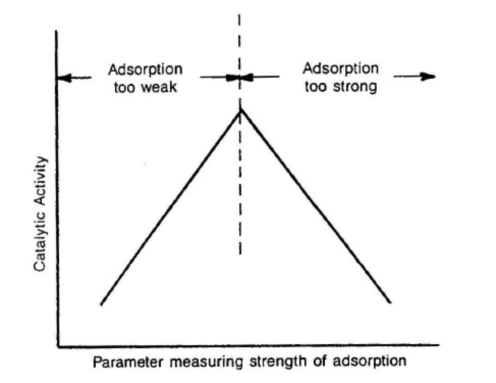
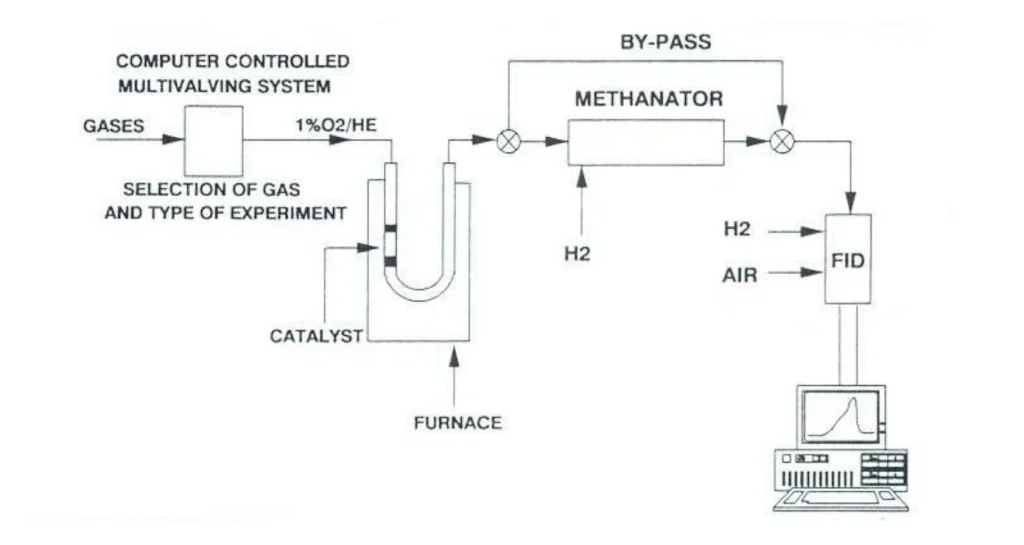
Advanced catalyst characterization through temperature-programmed analysis and dynamic chemisorption methods […]
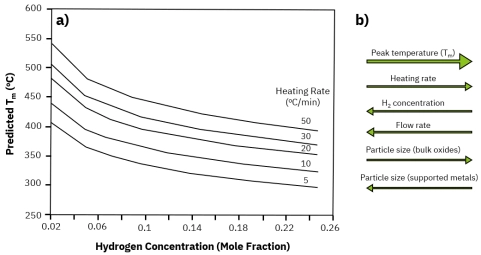
Direct comparison of TPR data is challenging because key experimental parameters—such as heating rate, hydrogen concentration, gas flow rate, and particle size—can significantly shift the reduction peak temperature (Tm) and alter the profile shape. Without matching or normalizing these conditions, differences in TPR profiles may reflect experimental setup rather than intrinsic catalyst reducibility.