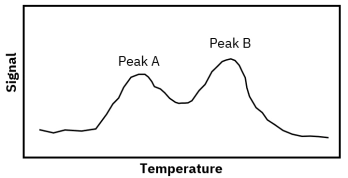
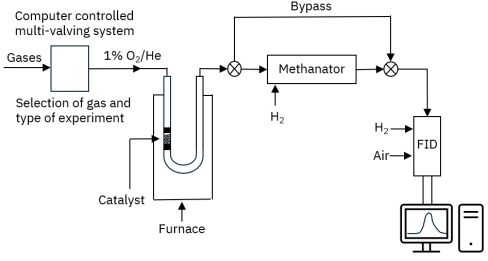
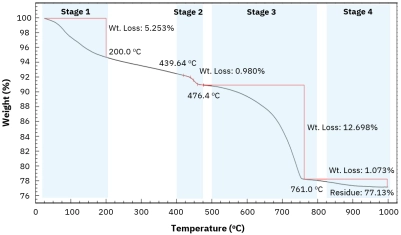
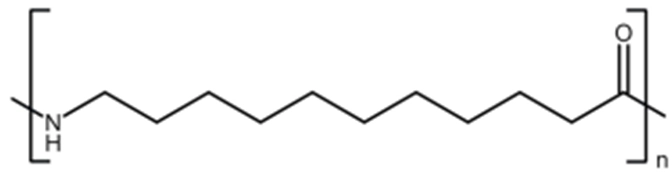
Understanding TPD kinetics, surface characterization, and desorption dynamics for heterogeneous catalyst analysis.[…]
Modern benchtop XRD systems provide rapid analysis (15–30 minutes per scan), high-resolution data, and compatibility with Rietveld refinement software. They allow battery research labs to perform phase identification, structural tracking, and quality control without relying on synchrotron facilities. This makes them cost-effective and highly suitable for continuous cathode material development programs.