Heterogeneous catalysis is integral to a wide array of industrial applications, including energy, chemical synthesis, and consumer products. Traditionally, a solid or powder catalyst is employed to transform gas phase hydrocarbons into valuable products. Elemental carbon deposition onto the catalyst, or “coking,” is an undesirable side reaction that, over time, will block the catalytic sites and deactivate the catalyst. Therefore, characterization of carbon deposits is essential for improving catalyst performance. Today, advanced techniques such as transmission electron microscopy (TEM), laser Raman spectroscopy, electron energy loss spectroscopy (EELS), solid-state 13C NMR, and temperature-programmed oxidation (TPO) are widely used to study coked catalysts. Among these, TPO has become one of the most commonly applied methods due to its simplicity and effectiveness.(1)
Temperature-programmed oxidation (TPO) is a materials characterization technique in which the sample is exposed to oxidizing gas, and the oxidizer chemically binds (chemisorbs) onto the surface. As the material temperature is increased, the oxidized surface species desorb from the material and are analyzed by a detector. For coke analysis, the catalyst is heated under O2 flow, and surface carbon is oxidized to CO2. The amount of desorbed CO2 is directly related to the amount of coke, and the temperature at which CO2 desorbed can differentiate between types of carbon on the catalyst. Typically, the desorbed product is analyzed by a thermal conductivity detector (TCD) or flame ionization detector (FID), but neither detector is sufficiently sensitive to CO2. Therefore, a methanation step can be employed to convert CO2 into CH4, which is easily measured by a flame ionization detector (FID). This process is shown in Scheme 1.
This AMI Note discusses the use of TPO combined with an innovative detection method developed by Dr. S.C. Fung and Dr. C.A. Querini at Exxon Research and Engineering Company.(2)This approach is straightforward and enables continuous monitoring of the rate of
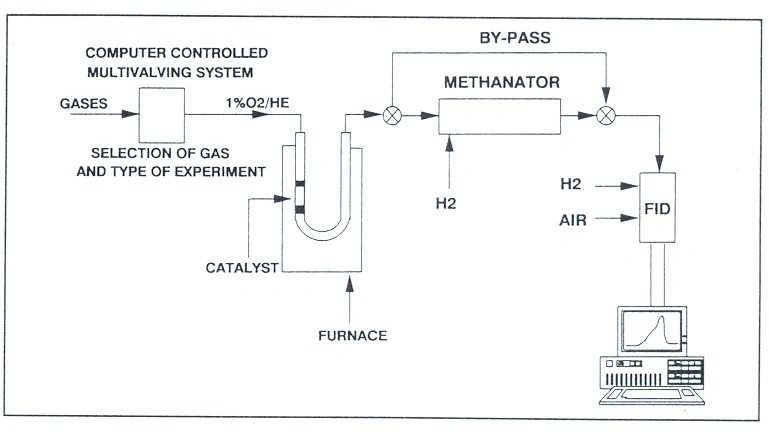
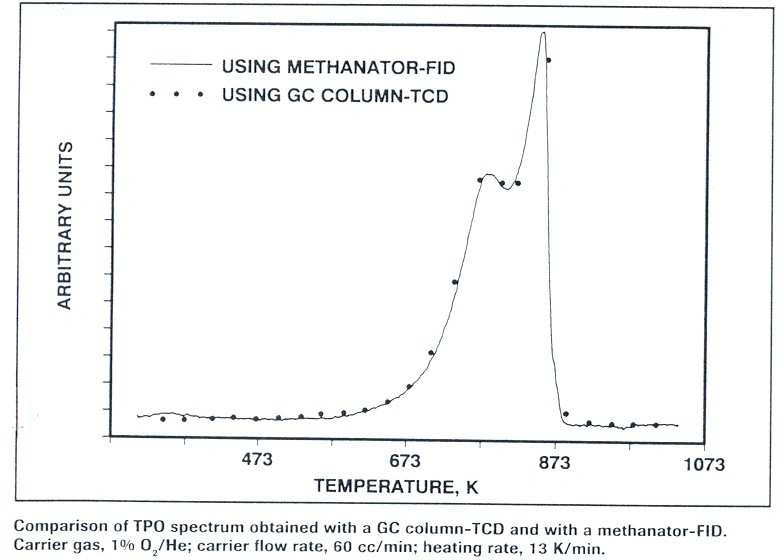
For these experiments, approximately 20 mg of coked catalyst were loaded into the sample cell. A helium carrier gas containing a low concentration of oxygen flowed over the sample at a rate of 20–80 cc/min. The temperature was increased linearly from room temperature until the complete oxidation of all carbon deposits.
The methanator contained approximately 500 mg of 40 wt% Ru/zeolite 13X. A pure hydrogen stream was injected into the methanator at a flow rate of 22 cc/min. Under these conditions, CO₂ was quantitatively converted to CH₄, while the oxygen in the carrier gas was reduced to water. The combined gas stream then flowed directly into the FID.
The FID continuously monitored the methane generation rate, which was equivalent to the rate of coke oxidation.
Influence of Oxygen Concentration, Flow Rate, and Methanator Temperature
Since TPO experiments require an excess of oxygen, it was necessary to evaluate how oxygen concentration affects the hydrogenation of CO₂ to CH₄ and to establish optimal operating conditions. The effects of flow rate and methanator temperature on the hydrogenation efficiency of the ruthenium catalyst were also investigated, along with the impact of various pretreatments on the Ru catalyst’s activity.
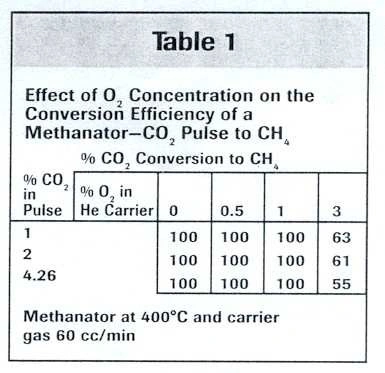
To study the influence of oxygen concentration, pulses of 1%, 2%, and 4.26% CO₂ in helium were introduced into the methanator using a pure helium carrier. Additional CO₂ pulses were introduced using helium carriers containing 0.5%, 1%, and 3% oxygen.
As shown in Table 1, the CO₂ pulses were completely converted to CH₄ except when the oxygen concentration was increased to 3%. One possible explanation for this behavior is that water formed in the methanator (due to the oxygen present in the carrier gas) reduced the equilibrium conversion of CO₂ to CH₄.
CO₂ + 4H₂ → CH₄ + 2H₂O
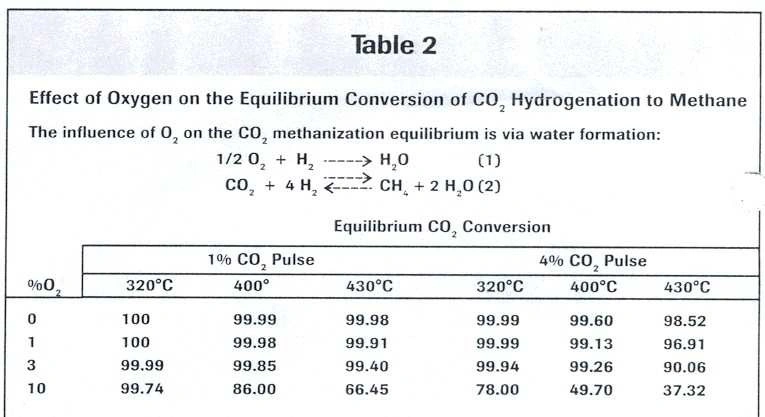
Higher oxygen concentrations lead to greater water formation, which in turn affects the equilibrium conversion of CO₂ to CH₄. Table 2 shows that TPO experiments should be conducted with oxygen concentrations at or below 3% and at methanator temperatures below 430°C to avoid equilibrium limitations. However, the incomplete conversion of CO₂ to CH₄ observed in Table 1 is not attributable to equilibrium constraints.
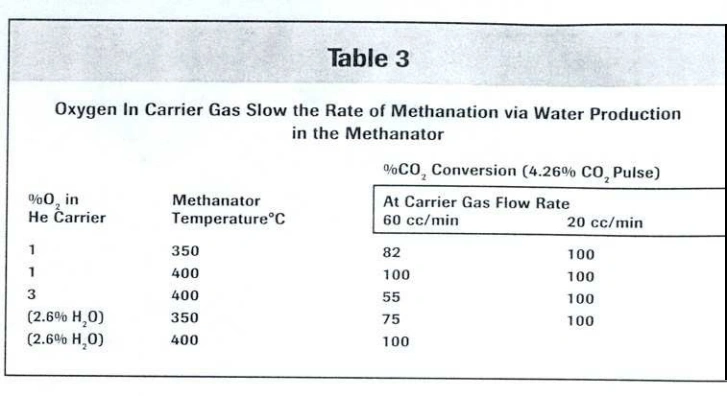
An alternative explanation is that water inhibits the methanation activity of the ruthenium catalyst. To investigate this, experiments were conducted varying three parameters: the oxygen concentration in the carrier gas, the methanator temperature, and the carrier gas flow rate. As shown in Table 3, it was necessary to reduce the flow rate when higher oxygen concentrations were used
Additional experiments introduced water directly into the system by saturating the carrier gas at room temperature, producing a 2.6% water concentration in helium. This water level is comparable to that generated during oxidation in a 1.3% oxygen environment. These results confirmed previous findings that oxygen concentrations should ideally remain below 2%. For experiments requiring higher oxygen levels, an oxygen trap can be installed upstream of the methanator. These traps effectively remove oxygen without affecting the CO₂ concentration exiting the sample U-tube.
Temperature-Programmed Methanation Studies
Temperature-programmed methanation experiments were also performed to evaluate the stability of the ruthenium catalyst and to determine the optimal methanator temperature for converting CO₂ to CH₄.
As indicated in Table 3, CO₂ conversion increased with both rising temperature and decreasing flow rate. This behavior suggests that conversion limitations in the presence of oxygen are primarily kinetic in nature. Additionally, the catalyst’s activity improved with temperature. However, the methanator temperature should be kept as low as possible to minimize the risk of sintering the ruthenium particles, which would permanently reduce catalyst activity.
Effect of Carrier Gas Flow Rate and Catalyst Stability
Experimental results indicated that FID sensitivity increased linearly with carrier gas flow rates up to 60 cc/min. At higher flow rates, the FID response plateaued, suggesting that flow rates above this level do not further improve sensitivity.
An additional important observation was the deactivation of the ruthenium catalyst in the methanator due to sulfur poisoning. This deactivation was caused by sulfur oxides generated during the combustion of sulfur-containing coke deposits. The most effective solution was the installation of a sulfur oxide trap upstream of the methanator, which successfully removed sulfur contaminants without affecting the CO₂ concentration.
Conclusions
These experiments demonstrate that TPO coupled with methanation and FID detection is a highly effective technique for monitoring the carbon oxidation rate of coked catalysts. By optimizing experimental parameters, complete conversion of CO₂ or CO to CH₄ is achievable, even in the presence of oxygen-containing carrier gases.
This method is sensitive enough to detect carbon concentrations below 0.1% and can distinguish subtle variations in the coke distribution on catalyst surfaces.
This Altamira Note summarizes a presentation delivered by Dr. S.C. Fung at an AMI (formally: Altamira’s) U.S. User’s Meeting. For further details on this TPO methodology, see: S.C. Fung and C.A. Querini, “A Highly Sensitive Detection Method for Temperature-Programmed Oxidation of Coke Deposits: Methanation of CO₂ in the Presence of O₂,” Journal of Catalysis, 138, p. 240 (1992).