Advanced Measurement Instruments
Copyright © 2025 Advanced Measurement Instruments
Overview
Conventional differential scanning calorimetry (DSC) can be used to quantify the amount of one crystalline form in a mixture of two forms—even when thermal transitions are not fully resolved. The compound studied has two crystalline anhydrous forms that melt at 173 °C and 189 °C, with heats of fusion of 29.3 kJ/mol and 26.4 kJ/mol, respectively.
The AMI DSC 600, with its high sensitivity, baseline stability, and flexible heating control, enables precise quantification of overlapping thermal events critical for polymorph analysis.
DSC Behavior of the Pure Forms
A sample composed entirely of the lower-melting form behaves differently depending on the heating rate:
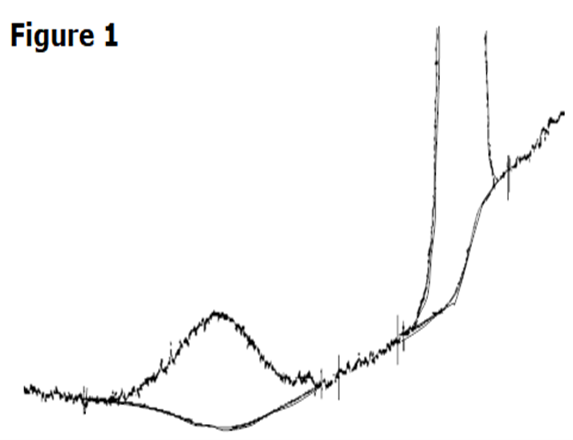
At fast heating rates (e.g., 10 °C/min) – Figure 2, the conversion does not complete in time. Instead, the lower-melting form:
This results in a thermal curve with an initial melting endotherm, an overlapping crystallization exotherm, and a second melting endotherm.
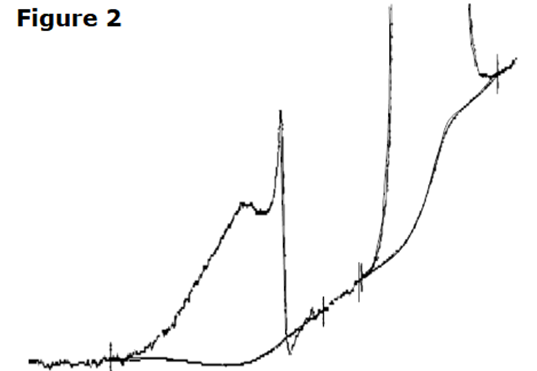
A sample comprised entirely of the higher-melting form shows a single melting peak, without intermediate transitions.
DSC Behavior of Mixtures and Quantification
Samples containing mixtures of both polymorphs typically produce thermal curves that resemble those of the lower-melting form. Depending on the heating rate, they may show combinations of solid-state transition, melting, and recrystallization. The key analytical parameter is the net enthalpy of the full transformation process.
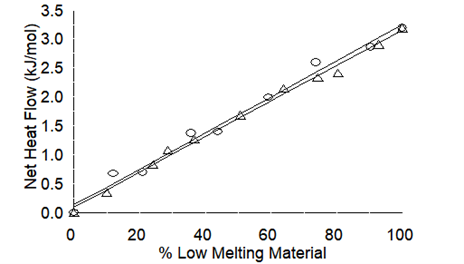
According to the first law of thermodynamics, the total energy to convert the lower-melting form to the higher-melting form is constant, regardless of the mechanism. For mixtures, this net energy is directly proportional to the amount of the lower-melting material – Figure 3
The AMI DSC 600 allows for accurate integration of these overlapping transitions, enabling reliable quantification even when peaks are not fully resolved.
Conclusion
The AMI DSC 600 enables quantitative analysis of polymorphic mixtures using conventional DSC methods, even when transitions are complex or overlapping. By measuring net enthalpy across the transformation sequence, users can determine the relative proportions of polymorphs with confidence.
This capability is especially useful in pharmaceutical development, materials science, and quality control applications—where understanding polymorph content can be critical to product performance and stability.