Advanced Measurement Instruments
Copyright © 2025 Advanced Measurement Instruments
Choice of Adsorbate
The choice of adsorbate is critical in temperature-programmed desorption (TPD) experiments. The selected gas should chemisorb selectively on the metal, avoiding sorption on the support or other catalytic components. Ideally, the adsorbate should form a stable monolayer and avoid irreversible reactions with either the metal or the support.
| Metal | Adsorbate(s) | Comments |
|---|---|---|
| Fe | H₂, CO | CO may form various carbonyls or carbides |
| Co | H₂, CO | Activated chemisorption; temperatures above 100°C are necessary for full coverage |
| Ni | H₂ | Rapid equilibration; CO forms Ni(CO)₄ |
| Cu | N₂O, Cl₂ | Involve surface reactions |
| Ru | H₂ | CO forms Ru(CO)₅; forms volatile oxide |
| Rh | H₂, CO | H₂ and CO stoichiometries vary with crystallite size |
| Pd | CO | H₂ can dissolve into the metal and form the bulk hydride |
| Re | H₂, O₂ | H₂ uptake can be low; forms volatile oxide |
| Os | H₂, O₂ | H₂ uptake can be low; forms volatile oxide |
| Ir | H₂, CO | H₂ and CO stoichiometries vary with crystallite size |
| Pt | H₂, O₂, CO | Activated H₂ chemisorption; CO stoichiometry can vary; CO can disproportionate on the metal at high temperatures |
| Mo, W | O₂ | Low temperatures required |
| Ag, Au | O₂ | High temperatures (420-570 K) required |
Table 1: suitable adsorbates
Example: CO can react with nickel to form volatile—and hazardous—nickel carbonyl (Ni(CO)₄),
making it unsuitable for certain Ni catalyst systems.
The adsorbate–metal interaction can be visualized using potential energy diagrams (Figure 1).
The initial physisorption step involves a small activation barrier (ΔE₁) and a minor energy release
(ΔH₁). Transitioning to a chemisorbed state requires overcoming a second activation barrier,
which may be small (ΔE₂) or large (ΔE₃). The heat of chemisorption (ΔH₂) is independent of this
barrier.
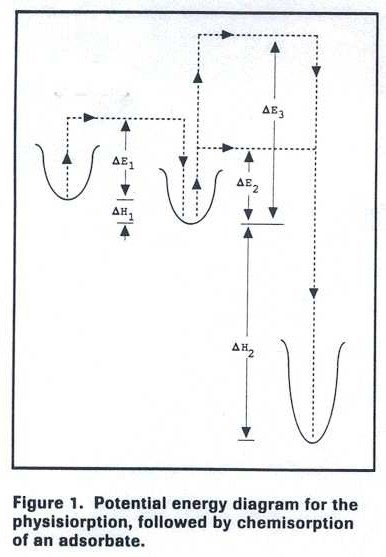
If the activation barrier is high, the process is classified as activated chemisorption, which
proceeds slowly and may require higher temperatures or longer adsorption times for full surface
coverage.
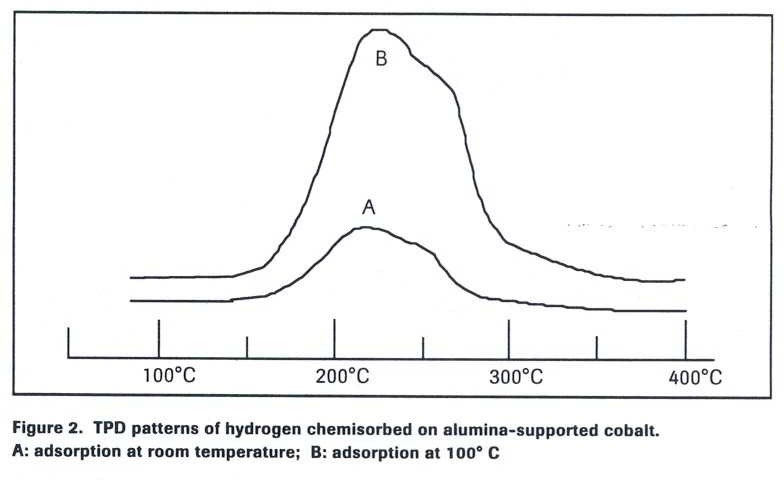
Case Example:
Hydrogen chemisorption on supported cobalt metal is activated. As shown in Figure 2:
full site coverage and a significant TPD signal.
Because theoretical guidance is limited, adsorbate selection typically relies on literature
precedent and practical experience. Table 1 provides a summary of suitable adsorbates for
common catalytic metals.
Choice of
Adsorption Conditions
Adsorption conditions must balance complete surface coverage with minimal side reactions.
Key Considerations:
undesirable reactions (e.g., CO disproportionation to CO₂ and carbon).
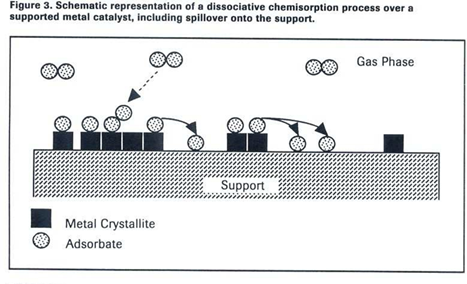
Spillover Warning:
Spillover, where adsorbates migrate from metal crystallites to the support (see Figure 3), is a
kinetically slow process that can distort TPD data if adsorption times or temperatures are too
high.
Recommended Starting Conditions:
Adsorbate Stoichiometry
Interpreting TPD results requires knowledge of the adsorbate-metal stoichiometry. While direct
measurement is not possible in simple TPD experiments, stoichiometry can be estimated by:
General Rules:
In some cases (e.g., very small Rh or Ir crystallites), H₂/M stoichiometries of 1 have been reported,
but these are rare.
When precise stoichiometry cannot be determined, CO uptake can still be used as a relative basis
for comparing catalysts.
Advanced Stoichiometry Determination Using the AMI-300IR
For IR-active adsorbates (such as CO, NO, and selected hydrocarbons), the AMI-300IR provides a
superior method for stoichiometry determination.
By integrating in-situ IR spectroscopy with TPD and chemisorption analysis, the AMI-300IR
enables:
Example:
During CO adsorption, the AMI-300IR can distinguish linear CO on atop sites from bridged CO
species. This capability enhances stoichiometric precision and provides deeper insight into
adsorption mechanisms.
The AMI-300IR is particularly valuable for:
References