In previous issues of AMI notes, we discussed the application of dynamic techniques—specifically, pulse chemisorption and temperature-programmed desorption […]
In previous issues of AMI notes, we discussed the application of dynamic techniques—specifically, pulse
chemisorption and temperature-programmed desorption—for catalyst characterization. A frequently asked
question is how results from these dynamic methods compare with those obtained from static volumetric
chemisorption. This note addresses that question by comparing data collected for various catalysts using
both static and dynamic approaches.
Pulse Chemisorption
This technique is particularly well-suited for catalyst-adsorbate systems with relatively fast adsorption
kinetics, meaning the adsorption process is not activated.
For this study, we selected two supported platinum (Pt) catalysts with low metal loading:
Carbon monoxide (CO) used as the adsorbate.
While hydrogen, CO, and occasionally oxygen are commonly used adsorbates for static (volumetric)
chemisorption of Pt catalysts [2-5], hydrogen adsorption on Pt exhibits slow kinetics under dynamic
conditions, making it a less suitable choice. Conversely, CO adsorption on Pt is well-established as a rapid
process, making CO the ideal choice for pulse chemisorption experiments.
Results
Table 1 presents a comparison of CO uptake measurements for the two Pt catalysts using both volumetric
and pulse techniques. The results show excellent agreement between the methods. Notably, the pulse
method yielded slightly lower uptake values, likely because the volumetric method also captures weakly
held or “reversible” CO that the pulse technique does not detect.
| Pt Chemisorption Data | |||
|---|---|---|---|
| Catalyst Description | Pt Loading (wt%) | Method | CO Uptake (µmol/g catalyst) |
| ASTM Standard Pt/Al₂O₃ | 0.5 | Volumetric Chemisorption | 10.2 |
| ASTM Standard Pt/Al₂O₃ | 0.5 | Pulse Chemisorption | 8.8 |
| In-house Pt/Al₂O₃ | 0.3 | Volumetric Chemisorption | 10.0 |
| In-house Pt/Al₂O₃ | 0.3 | Pulse Chemisorption | 9.1 |
Advantages of the Pulse Method
One significant advantage of the pulse chemisorption method over the volumetric approach is the time
required to complete the analysis. After the necessary pretreatment—which is similar for both techniques—
a full pulse chemisorption experiment typically takes less than 30 minutes, including system calibration.
In comparison, a standard five-point volumetric measurement can easily require six hours or more.
A second advantage is the ease with which the sensitivity of the pulse method can be enhanced. This can be
achieved by using smaller pulse loops or by diluting the adsorbate in an appropriate inert carrier gas. For
example, a 10% CO in helium mixture can replace pure CO. While this may slightly increase the duration of
the analysis, it significantly improves reproducibility.
Temperature-Programmed Desorption (TPD)
Temperature-programmed desorption (TPD) is especially useful when catalyst–adsorbate kinetics are not
favorable for pulse chemisorption measurements (see Altamira Notes No. 19, Winter 1994) [6]. Cobalt metal
catalysts are a typical example. For supported cobalt catalysts, hydrogen chemisorption proceeds slowly at
room temperature but can be accelerated by raising the sample temperature to approximately 100°C [7].
Carbon monoxide also chemisorbs slowly at room temperature and poses an additional challenge: at
elevated temperatures, CO can disproportionate via the Boudouard reaction:
2CO→C+CO2
In this study, the hydrogen uptake of two cobalt catalysts was measured using both volumetric
chemisorption and TPD. The catalysts contained 20 wt% Co supported on alumina; one catalyst also
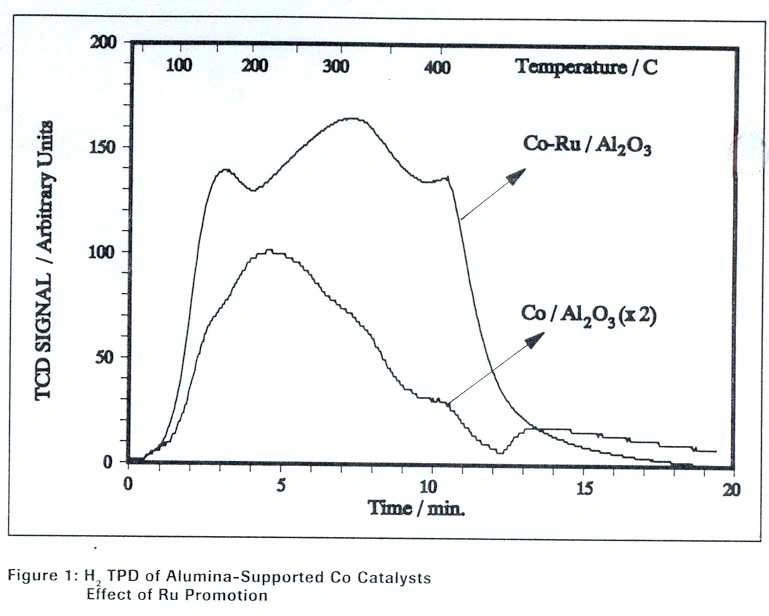
included 0.5 wt% ruthenium as a promoter to aid reduction and improve dispersion. Figure 1 presents the
hydrogen TPD profiles of these catalysts. Both exhibited broad desorption profiles, with the Ru-containing
catalyst showing a larger desorption signal, as expected. The key parameters for obtaining reliable TPD
results were an adsorption temperature of 50°C and an adsorption time of 30 minutes.
Table 2 summarizes the hydrogen uptake measured by both TPD and a five-point volumetric method. Once
again, excellent agreement was observed between the two techniques.
As with pulse chemisorption, TPD offers significant time savings compared to traditional volumetric
methods. Furthermore, TPD provides valuable qualitative information about the strength of chemisorption
based on the temperature distribution of the desorption profile.
| Co-based Catalyst H₂ Uptake | |||
|---|---|---|---|
| Catalyst Description | Promoter | Method | H₂ Uptake (µmol/g catalyst) |
| 20 wt% Co/Al₂O₃ | None | Volumetric Chemisorption | 41 |
| 20 wt% Co/Al₂O₃ | None | TPD | 42 |
| 20 wt% Co/0.5 wt% Ru/Al₂O₃ | Ru | Volumetric Chemisorption | 164 |
| 20 wt% Co/0.5 wt% Ru/Al₂O₃ | Ru | TPD | 188 |
Summary
This study demonstrates that both dynamic (pulse chemisorption and TPD) and static (volumetric)
chemisorption techniques, when properly applied, yield consistent and reliable results across a variety of
catalysts. Dynamic methods offer clear advantages in terms of simplicity, flexibility, and significantly
reduced analysis time—often cutting hours down to minutes. Additionally, dynamic techniques provide
enhanced sensitivity and the potential for gaining qualitative insights into adsorption strength and surface
interactions, making them ideal for both research and quality control environments.
References