Advanced Measurement Instruments
Copyright © 2025 Advanced Measurement Instruments
Introduction
Chemisorption—the formation of chemical bonds between gas-phase molecules and surface atoms—is the foundational step in heterogeneous catalysis. On supported metal catalysts, this process occurs on small metal crystallites anchored to high surface area oxide materials. These chemisorbed species react with additional adsorbed molecules or gas-phase reactants to generate catalytic products.
The chemisorption behavior of a catalyst directly impacts both the reaction rate and selectivity toward desired products. Understanding and quantifying chemisorption is thus essential for both catalyst design and performance optimization.
The Link Between Chemisorption and Catalysis
Optimal catalytic performance requires a balance between the strength and quantity of chemisorbed species:
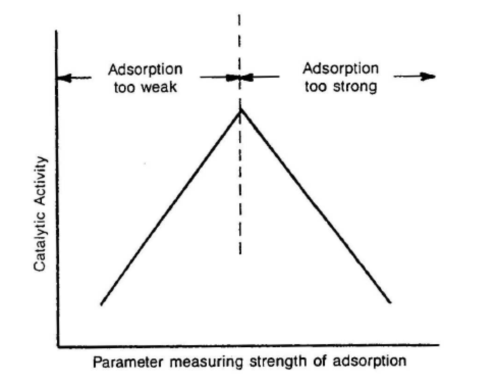
Figure 1: Volcano Curve
Measuring Chemisorption
Quantitative assessment of chemisorption requires techniques that can evaluate both the number and strength of adsorption sites:
| Method | Measurement Focus |
| Static/Volumetric Chemisorption | Equilibrium uptake of gas molecules (closed system). |
| Pulse Chemisorption | Uptake of calibrated gas pulses. |
| Temperature-Programmed Desorption (TPD) | Desorption behavior upon heating — provides both site count and adsorption strength. |
Among these, TPD offers the most comprehensive data, capturing both quantitative and qualitative characteristics of chemisorption sites.
Temperature-Programmed Desorption (TPD): Principles and Procedure
A standard TPD experiment involves:
Example:
For H₂ chemisorption on Ni/SiO₂ (H₂:Ni = 1:2), TPD reveals both the number of available Ni sites and the strength distribution of hydrogen binding.
AMI Solutions: Automated Chemisorption Analysis
AMI Chemisorption Analyzers automate the entire process:
The AMI platform delivers reproducible, operator-independent TPD and chemisorption measurements, empowering researchers to optimize catalysts and advance reaction engineering.
Conclusion
Chemisorption is not just a surface phenomenon—it is the gateway to catalytic function. Through advanced, automated analysis tools like the AMI Chemisorption Series, scientists can quantify and understand the key properties driving catalyst performance.