Advanced Measurement Instruments
Copyright © 2025 Advanced Measurement Instruments
Electronic specialty gases are essential foundational materials in modern electronics manufacturing—often referred to as the "blood" or "food" of the industry. These high-purity gases are critical to the production of semiconductors, display panels, LEDs, and photovoltaics. In 2021, the demand from these sectors accounted for 43%, 21%, 13%, and 6% of total consumption, respectively [1].
The purity requirements for electronic specialty gases are stringent, typically at the 5N (99.999%) level, with some applications demanding 6N (99.9999%) or even higher. Gas purity and quality directly influence device yield and performance, making both synthesis and purification technologies central to specialty gas production. Common synthesis methods include electrolysis, chemical reactions, and combined electrochemical approaches, while purification techniques span adsorption, distillation, absorption, and membrane separation [1].
Semiconductor manufacturing, comprising thousands of highly specialized steps, relies on over 100 types of specialty gases—44 of which are commonly used. These include compounds such as trifluorine nitride, tetrafluorocarbon, and sulfur hexafluoride, typically supplied in liquid or pressurized gas form.
Each major semiconductor process uses specialty gases for critical roles:
These processes demand precise control and validation of gas delivery, purity, and usage conditions—making gas analysis, gravimetric sorption, and breakthrough testing indispensable tools in quality assurance and process development.
The adsorption method leverages the principle that porous materials exhibit selective gas uptake depending on the molecular properties of the gas and the characteristics of the material. For a given porous adsorbent, different gases exhibit different adsorption capacities. When a gas mixture is passed through an adsorbent bed, gases with stronger affinities to the surface will be preferentially adsorbed, while those with weaker interactions will exit the system—achieving a separation effect. The adsorbed gas can subsequently be desorbed by thermal regeneration or gas purging, allowing for recovery of purified components [1].
One prominent application of this technique is the separation of xenon (Xe) and krypton (Kr)—a challenging and high-value target for gas purification. Xe and Kr are critical to industries such as semiconductors, medical imaging, aerospace, and lighting.
Wang et al. developed a negatively charged coordination ultramicroporous material, NbOFFIVE-2-Cu-i (ZU-62), that demonstrated a breakthrough in Xe/Kr separation performance [3]. ZU-62 features a finely tunable pore size and flexible framework, resulting in a unique inverse size sieving effect—a rare case where the larger atom (Xe) is selectively adsorbed over the smaller atom (Kr). As shown in FIGURE 1, static adsorption isotherms confirm high Xe uptake and preferential exclusion of Kr due to the material’s precise pore chemistry and geometry.
To validate the material's real-world separation efficiency, dynamic breakthrough experiments were performed at 273 K (FIGURE 2). Kr eluted early, while Xe showed delayed breakthrough—demonstrating strong selective adsorption of Xe. The system achieved production of >99.9% pure krypton from the effluent stream and record Xe capture of 206 mL/g (2.88 mmol/g), aligning well with isotherm data and confirming the industrial relevance of the material [3].
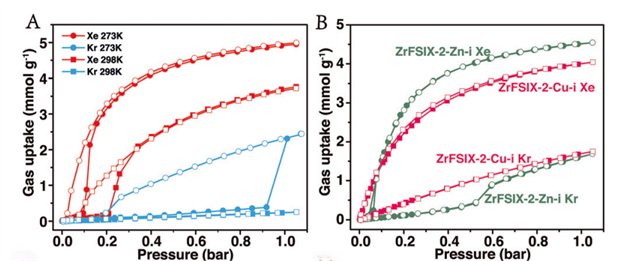
Figure 1 Adsorption and desorption isotherms of pure components of Xe and Kr by ZU-62 at 273 K and 298 K
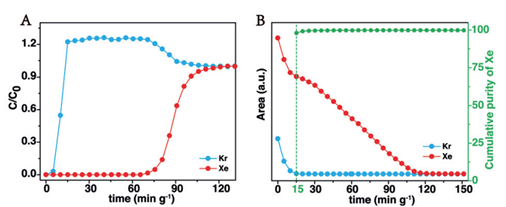
Figure 2 (A) Pore penetration experiment of Xe/Kr (20/80) mixture at 273 K and 1 bar conditions; (B) Desorption signals of Xe and Kr during regeneration process at 298 K with a flow rate of 3.5 mL/min-1 of N2, the green curve represents the real-time cumulative purity of Xe.
Sample Selection and Objective
Two metal-organic framework (MOF) materials were selected for adsorption and gas separation studies:
Both dynamic breakthrough experiments and static isotherm measurements were performed to evaluate the adsorption capacity and selectivity of each material under relevant conditions.
Dynamic Breakthrough Testing
Competitive adsorption experiments were performed using the BTSorb-100 Breakthrough Curve and Mass Transfer Analyzer from AMI. A 1 mL stainless steel adsorption column (inner diameter: 0.45 cm; bed height: 5 cm) was used for all tests.
Breakthrough curves were recorded and used to assess gas retention times, separation resolution, and dynamic capacity for each system.
Static Adsorption Testing
Static adsorption isotherms were measured using the AMI Sync 400 Surface Area and Pore Size Analyzer. For both MOFs:
The resulting data enabled comparison between static adsorption capacity and dynamic performance, offering insight into material structure-performance relationships.
Perfluorinated electronic specialty gases—including NF₃, CF₄, and SF₆—play a critical role in the fabrication of silicon-based semiconductors due to the strong chemical reactivity between fluorine and silicon. However, the conversion efficiency of F-gases in plasma processes is often below 60%, leaving unreacted F-gases and byproducts such as N₂, NOₓ, HF, and H₂O in the exhaust stream. As environmental regulations tighten and gas recovery becomes increasingly important, adsorbent-based purification has emerged as a key area of research and development.
To assess adsorption-based purification of SF₆, MOF-1 was tested for its separation capability in an SF₆/N₂ gas mixture.
Static Adsorption Behavior
As shown in FIGURE 3(a), static adsorption isotherms indicate that MOF-1 exhibits a significantly higher adsorption capacity for SF₆ compared to N₂. This disparity suggests the potential for selective adsorption and separation of SF₆ from nitrogen under controlled conditions.
Dynamic Breakthrough Performance
To evaluate real-world separation behavior, a dynamic breakthrough experiment was conducted using the BTSorb-100 system. A 10:90 SF₆/N₂ gas mixture was introduced at 298 K, 2 bar, and a total flow rate of 30 mL/min.
As shown in FIGURE 3(b), the breakthrough curve illustrates clear separation behavior:
Calculated dynamic adsorption capacities were:
These results were consistent with the static isotherm values, where MOF-1 adsorbed 1.01 mmol/g of N₂ at 95 kPa and 0.896 mmol/g of SF₆ at 9.28 kPa.
The close agreement between static and dynamic data confirms the reliability and practical relevance of the measured separation behavior. Taken together, these findings demonstrate that MOF-1 offers viable adsorption and separation performance for SF₆/N₂ systems, and highlights its potential use in semiconductor exhaust gas purification processes.
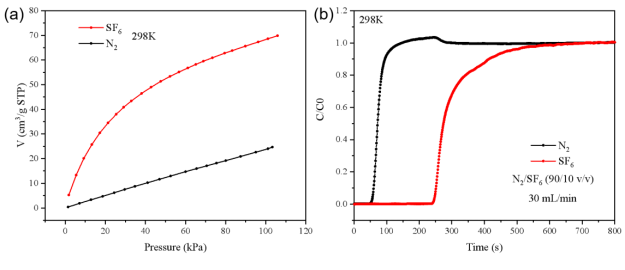
Figure 3 (a) Adsorption isotherms of SF6 and N2 on MOF-1 at 298K; (b) Competitive adsorption breakthrough curve of MOF-1 at a temperature of 298K, a total pressure of 2 bar, and a total flow rate of 30 mL/min (He/N2/SF6 = 50/45/5).
Xenon (Xe) and krypton (Kr) are high-value noble gases, often referred to as "golden gases" due to their scarcity and wide-ranging industrial applications. Their unique physical and chemical properties make them essential in fields such as semiconductor manufacturing, lighting, aerospace, medical imaging, and anesthesia [4]. As a result, the efficient separation and purification of Xe from Kr remains a high-priority challenge in specialty gas processing.
In this study, MOF-2 was evaluated for its ability to adsorb and separate Xe/Kr mixtures.
Static Adsorption Isotherms
As shown in FIGURE 4(a), adsorption isotherms measured at 273 K demonstrate that MOF-2 exhibits a significantly higher adsorption capacity for Xe compared to Kr. When the temperature was increased to 298 K (FIGURE 4(b)), the adsorption capacities of both gases decreased—consistent with exothermic physisorption behavior—but Xe remained more strongly adsorbed than Kr, suggesting favorable selectivity across a range of operating conditions.
These static results confirmed MOF-2’s potential for selective Xe adsorption from a Xe/Kr mixture.
Dynamic Breakthrough Testing
To evaluate separation performance under flow conditions, a competitive dynamic breakthrough experiment was conducted using a 20/80 (v/v) Xe/Kr binary gas mixture. The test was carried out at 298 K, 1 bar, and a total flow rate of 3 mL/min.
As shown in FIGURE 4(c):
Calculated adsorption capacities were:
The close agreement between static and dynamic data, along with the large differential in breakthrough times, confirms that MOF-2 offers strong selectivity and capacity for Xe over Kr. These results demonstrate MOF-2’s practical utility for Xe purification from Kr-containing gas mixtures, with potential applicability in industrial gas recovery and semiconductor process optimization.
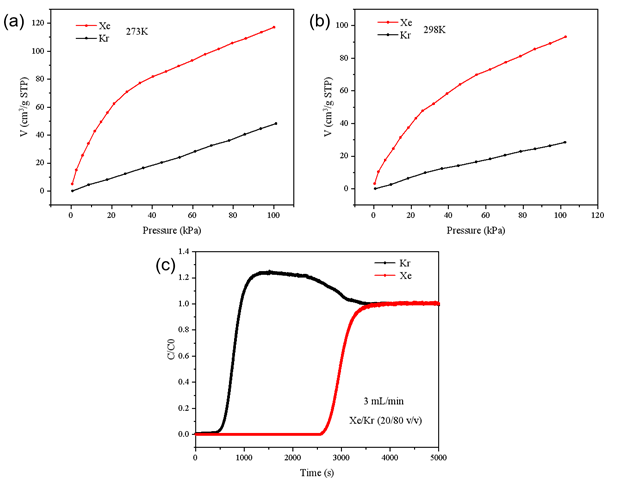
Figure 4 (a) Adsorption isotherms of Xe and Kr by MOF-4 at 273K; (b) Adsorption isotherms of Xe and Kr by MOF-4 at 298K; (c) Dynamic competitive adsorption curves of Xe/Kr by MOF-4 under normal temperature and pressure, total flow rate of 3 mL/min (Xe/Kr 20/80)
The results of this study confirm that advanced MOF materials offer strong potential for the selective adsorption and separation of high-value electronic specialty gases, including SF₆/N₂ and Xe/Kr mixtures. The close correlation between static isotherm data and dynamic
breakthrough results underscores the importance of combining both measurement approaches for accurate performance evaluation.
Using Sync 400 surface area and pore size analyzer and the BTSorb-100 breakthrough system, researchers were able to characterize adsorption behavior with high resolution and repeatability under realistic process conditions. These tools provided critical insights into both capacity and selectivity, enabling a comprehensive understanding of material performance.
As demand continues to grow for high-purity gases in semiconductor, medical, and aerospace industries, precision gas separation technologies will remain a key innovation area. AMI’s application-driven instrumentation supports the development and scale-up of advanced materials by delivering robust, accessible, and high-performance analytical solutions.
[1] He, H., Liu, Y., Zhang, J., et al. Research Progress in Synthesis and Purification Technologies of Electronic Specialty Gases. Low Temperature and Specialty Gases, 2023, 41(01): 1–5+46.
[2] China's Electronic Specialty Gases: From Import Substitution to Global Supply — In-depth Report on the Electronic Specialty Gas Industry.
[3] Wang, Q., Ke, T., Yang, L., Zhang, Z., Cui, X., Bao, Z., Ren, Q., Yang, Q., Xing, H. Angewandte Chemie, 2020, 132(9): 3451–3456.
[4] Zhao, Z. Research on Enhanced Xe/Kr Selective Adsorption Separation by Regulating Adsorbent Pore Environment. Nanchang University, 2023. DOI: 10.27232/d.cnki.gnchu.2023.003407.