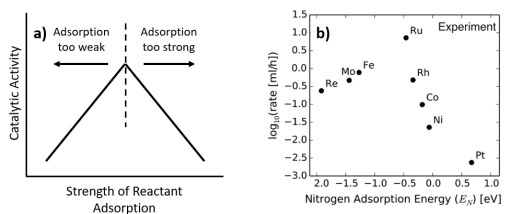
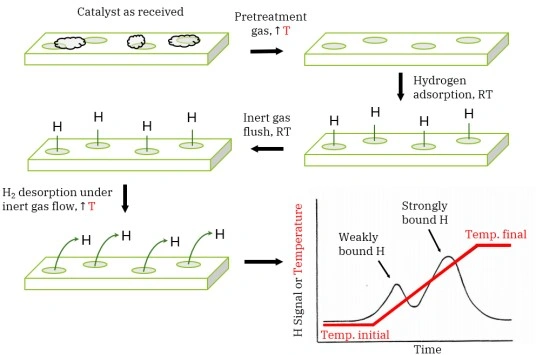
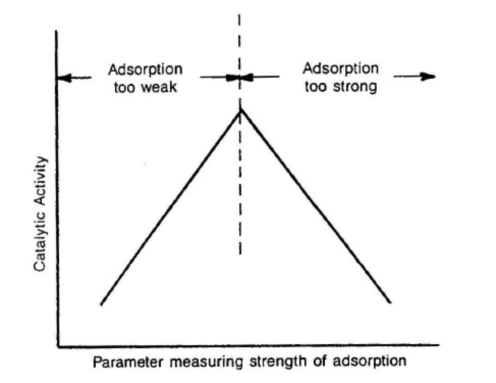
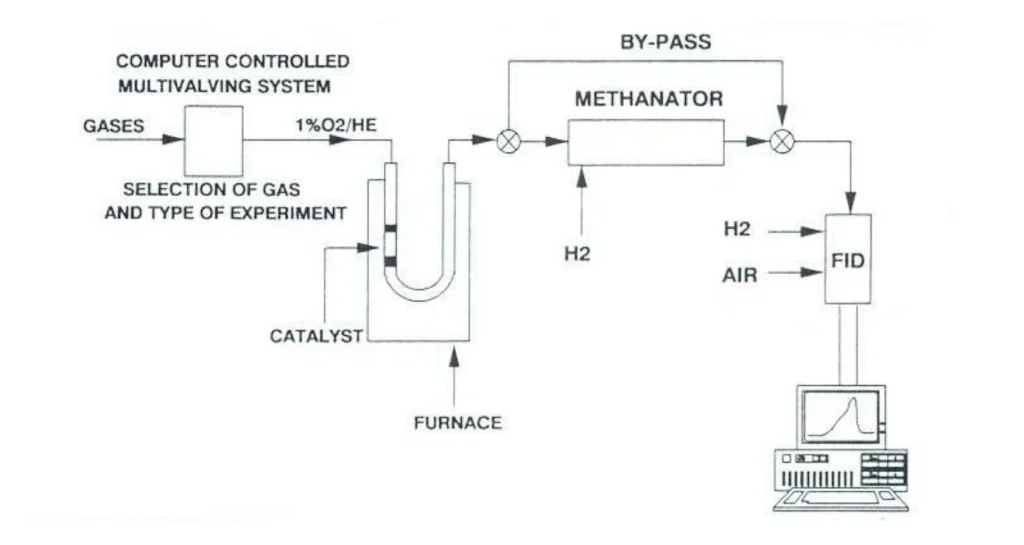
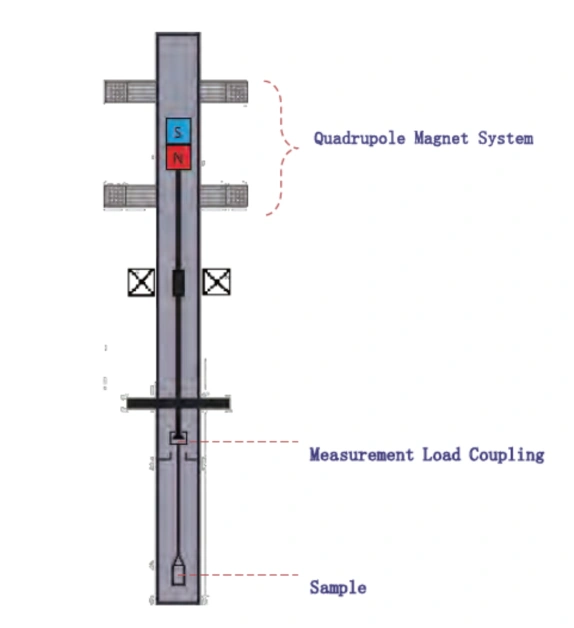
Chemisorption—the formation of chemical bonds between gas-phase molecules and surface atoms—is the foundational step in heterogeneous catalysis […]
Chemisorption analysis reveals key catalyst properties such as metal dispersion, number of active surface sites, adsorption strength, and metal-support interactions. These parameters help scientists optimize catalyst design for higher activity, selectivity, and long-term stability.