Cement is one of the most widely used construction materials across industries including infrastructure, transportation, agriculture, and marine engineering. Among cement […]
Background
Cement is one of the most widely used construction materials across industries including infrastructure, transportation, agriculture, and marine engineering. Among cement types, silicate cement holds a dominant role due to its versatility, durability, and long-standing industrial adoption.
The primary phase of silicate cement clinker comprises silicate minerals, with alite (C₃S) and belite (C₂S) accounting for over 75% of its composition. Upon exposure to atmospheric conditions, these components react with moisture and carbon dioxide to form secondary products such as:
Each of these phases decomposes at distinct temperatures, making thermogravimetric analysis (TGA) a powerful technique to identify and quantify cement hydration and carbonation products based on characteristic mass loss behavior.
Experimental Method
Thermogravimetric analysis was performed using the AMI TGA 1000 under the following conditions:
The analysis focused on the thermal decomposition behaviors of Ca(OH)₂ and CaCO₃, allowing their relative quantities to be determined based on water and CO₂ release, respectively.
Results and Discussion
Figures 1 and 2 present the TGA profiles for two silicate cement samples (Sample A and Sample B). The thermograms can be interpreted in four distinct mass loss stages:
Stage 1: < 200°C
Stage 2: 400–480°C
Stage 3: 500–800°C
Stage 4: > 800°C
Comparative Analysis: Sample A vs. Sample B
These results demonstrate that the AMI TGA 1000 provides excellent resolution for differentiating hydration and carbonation products in cement and allows for reliable quantitative analysis based on thermal decomposition behavior.
Conclusion
Thermogravimetric analysis using the AMI TGA 1000 enables clear and reliable identification of key hydration and carbonation phases in silicate cement. The system’s high sensitivity and stability allow differentiation between loosely and strongly bound components across a broad temperature range — from ettringite and Ca(OH)₂ to amorphous and crystalline CaCO₃.
The relative amounts of Ca(OH)₂ and CaCO₃ reflect hydration progress and carbonation extent, both of which impact durability and performance in real-world environments.
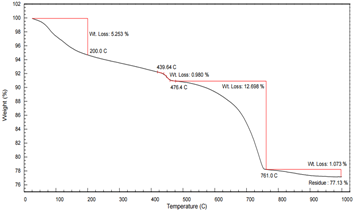
Figure 1: TGA of Silicate Cement Sample A
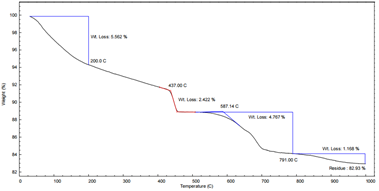
Figure 2: TGA of Silicate Cement Sample B