Advanced Measurement Instruments
Copyright © 2025 Advanced Measurement Instruments
Introduction
In many industrial gas separation processes, the presence of water vapor presents a major challenge. Whether in exhaust gas treatment or coalbed methane (CBM) recovery, moisture in the gas stream can severely degrade the performance of solid adsorbents. During CBM extraction, significant amounts of methane are mixed with air, forming low-concentration mixtures—over 70% of which are typically released directly into the atmosphere. Effective methane/nitrogen separation from these dilute streams offers both environmental and economic advantages.
However, water vapor often interferes with this separation, particularly in materials such as metal-organic frameworks (MOFs). These materials are known for their high affinity to water, which competes with target gases for active sites and can destabilize the framework structure. Understanding this competitive behavior is critical to optimizing performance—especially when selecting adsorbents for use in real-world environments.
MOFs in Humid Methane/Nitrogen Mixtures
To assess the impact of moisture on separation performance, tests were conducted on DMOF and DMOF-TM using a 50/50 CH₄/N₂ mixture under controlled relative humidity conditions.
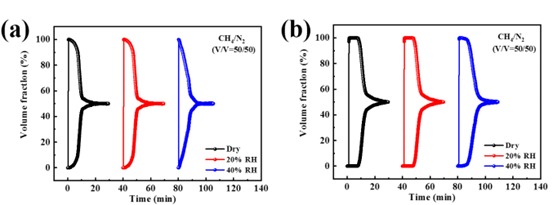
FIGURE 1: Permeation curves for CH₄/N₂ (50/50) at 298 K and 1 bar for (a) DMOF and (b) DMOF-TM at 20% and 40% RH
At 20% RH, both materials performed similarly to dry conditions. However, at 40% RH, DMOF failed to recover high-purity methane, while DMOF-TM exhibited earlier breakthrough and reduced selectivity. The decline is attributed to water’s competitive adsorption, which disrupts methane/nitrogen separation【1】.
With its ability to simulate real environmental humidity, AMI's BTSorb™ breakthrough system plays a vital role in quantifying this performance loss under humid conditions—enabling material screening that mirrors operational realities.
VOC Adsorption with Hydrophobic MOFs
Moisture also interferes with VOC removal. Hydrophobically modified UiO-66-NDC(50) shows decreasing toluene capacity with rising RH, from 143 mg/g at 0% RH to just 50 mg/g at 80%.
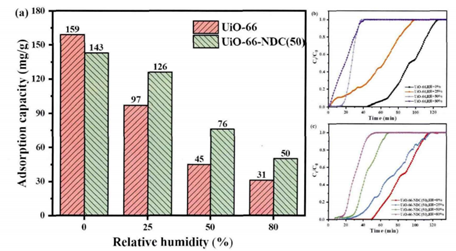
FIGURE 2: (a) Toluene adsorption capacity vs. humidity; (b)–(c) breakthrough curves for UiO-66-NDC(50)
Despite the presence of nonpolar functional groups, water molecules still dominate the adsorption landscape at high humidity. The ability to rapidly screen such performance drop-offs using AMI's modular vapor-generation capabilities gives researchers clear insight into material suitability【2】.
Ethylene Purification in Humid Gas Streams
In ethylene production, residual CO₂ and C₂H₂ must be removed to ultra-trace levels. Zeolite ETA-MOR is one candidate, but its performance suffers in humid conditions. However, after organic amine modification, ETA-MOR-0.5 maintains over 85% separation efficiency at 75% RH.
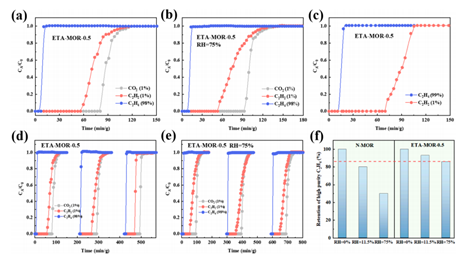
Figure 3: Permeation Curves for ETA-MOR Zeolite Molecular Sieve at 298 K (flow rate 5 ml/min); (a,b) Permeation Curves for ETA-MOR Zeolite in CO2/C2H2/C2H4 (1/1/98, v/v/v) Atmosphere under Dry and 75% RH Conditions; (c) Permeation Curve for ETA-MOR Zeolite in C2H2/C2H4 (1/1/99, v/v/v) Atmosphere; (d, e) Cycle Stability of ETA-MOR Zeolite in CO2/C2H4/C2H4 (1/1/98, v/v/v) Atmosphere under Dry and 75% RH Conditions; (f) Permeation Curves for Modified and Unmodified ETA-MOR at Different Humidities.
The amine modification alters the acid-base environment of the pores, enhancing hydrophobicity and reducing diffusion channels. AMI systems allowed for direct comparison of modified vs. unmodified materials across variable humidity conditions, helping pinpoint materials capable of high-selectivity operation under moisture stress【3】.
CO₂ Capture from Flue Gas with Humidity
Post-combustion CO₂ capture from flue gas—typically containing nitrogen, CO₂, and water vapor—is another key application where adsorbent performance must be tested under realistic humidity. While materials like NaX and EFS-10 degrade under moist conditions, functionalized sorbents such as EDA-Y and PEI/SiO₂ maintain strong performance due to the presence of amine groups that preferentially bind CO₂.
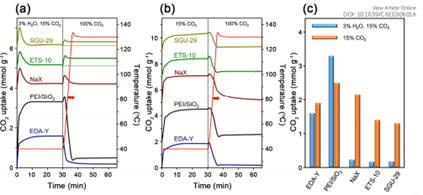
Figure 4: (a) TSA Adsorption-desorption Curve for CO2 (adsorption: H2O/CO2/Ar/N2 (3/15/2/80, v/v/v/v) at 313K, Desorption CO2 100, v@403K); (b) TSA Adsorption-desorption Curve for CO2 (CO2/Ar/N2 (15/2/83, v/v/v) at 313K, Desorption CO2 100, v@403K); (c) Comparison of CO2 Adsorption Amount at 3% Humidity and Dry conditions.
These results, made possible through AMI’s controlled-vapor testing infrastructure, demonstrate the need for precise experimental setups when evaluating adsorbents for use in flue gas environments【4】【5】.
Experimental Methods
Permeation and breakthrough experiments were carried out using AMI’s BTSorb™ 100 system.
Test Conditions:
With AMI’s advanced instrumentation, vapor content can be tightly regulated, enabling high-fidelity simulation of industrial scenarios.
Results and Discussion
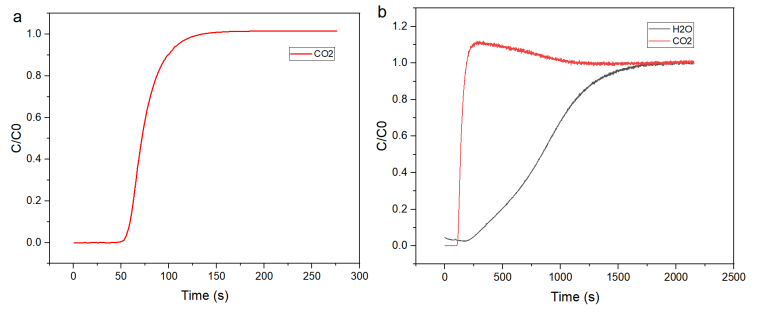
Figure 5 (a) Breakthrough Curves of the Molecular Sieve under Dry CO2/N2 (10/90, v/v) Atmosphere;
(b) Breakthrough Curves of the Molecular Sieve under CO2/N2 (10/90, v/v) Atmosphere with 80% Relative Humidity (RH=80%).
Under dry conditions, the CO₂ adsorption capacity reached 1.71 mmol/g, with a standard breakthrough curve. Under 80% RH, the capacity dropped to just 0.528 mmol/g due to water displacing CO₂ at the active sites. This competitive behavior would be missed using dry-gas-only evaluations—further underscoring the importance of humidity simulation during testing.
Conclusion
Water vapor is a critical factor affecting adsorption performance in gas separations. Whether for methane, VOCs, ethylene purification, or CO₂ capture, competitive adsorption by water significantly alters the effectiveness of many adsorbents.
AMI systems—equipped with integrated steam generators, configurable gas mixers, and real-time mass spec analysis—enable researchers and process engineers to test under realistic, application-specific conditions. This ensures more reliable data, better materials selection, and ultimately, more efficient gas separation processes.
References
[1] Li Tong. Study on Efficient Separation of Methane/Nitrogen under Humid Conditions Using DMOF Materials. Taiyuan University of Technology, 2022.
[2] Li Wenxiang. Hydrophobic Modification of MOFs (UiO-66) and VOC Adsorption Performance under Humidity. Shandong University, 2022.
[3] Shi X., Zhang B., Chen H. Organic Molecular Gate in Mordenite for Deep Removal of C₂H₂ and CO₂ from Ethylene. Sep. Purif. Technol., 2023.
[4] Mu J., Fang Z., Zhu H. Solid Adsorbents for CO₂ Capture in Flue Gas. Fine Chemicals, 2023, 40(9): 1857–1865.
[5] Cho H., Choi M., Sung S., et al. EDA-Grafted Y Zeolite: Regenerable CO₂ Adsorbent via TSA without Urea Formation. Energy Environ. Sci., 2016, 9(5): 1803–1811.