Advanced Measurement Instruments
Copyright © 2025 Advanced Measurement Instruments
Catalysts are at the heart of modern chemical manufacturing, with more than half of all chemical products depending on catalytic processes. Improving catalyst performance requires a deeper understanding of the physicochemical phenomena that occur at the catalyst–reactant interface, including diffusion, adsorption/desorption, surface reactions, and structural changes at the surface [1]. To investigate these processes, researchers study both the reaction mechanism and key kinetic parameters, such as surface coverage and reaction rates. One particularly powerful technique is SSITKA (Steady-State Isotopic Transient Kinetic Analysis), first introduced in the 1970s by Happel, Bennett, and Biloen.
SSITKA enables the measurement of kinetic parameters under true steady-state conditions. By rapidly switching from a reactant gas to its isotopically labeled analog—while maintaining constant flow, pressure, temperature, and catalyst surface state—SSITKA captures real-time information on surface intermediates, site coverages, and turnover rates without disrupting the reaction equilibrium. This application note reviews key literature examples of SSITKA in catalysis and highlights why AMI developed the AMI-200 then 300SSITKA—a fully integrated system designed to meet the growing demand for reliable, precise kinetic measurements.
SSITKA is a technique that rapidly switches from a reactant gas to its isotopically labeled counterpart under steady-state conditions. Using mass spectrometry, the system monitors transient response curves as unlabeled reactants and products decrease and labeled species increase. This enables the quantitative study of heterogeneous catalytic mechanisms and surface kinetics. The “steady state” ensures constant flow rate, pressure, temperature, surface coverage, and reactant/product concentrations throughout the switch—minimizing interference from isotopic effects [2]. Since most industrial catalytic processes operate under steady-state conditions, SSITKA provides highly relevant insights into reaction pathways, site coverages, and apparent activation energies. While early versions relied on radioactive isotopes, modern SSITKA systems now use stable isotopes such as ¹³C, ¹⁸O, ¹⁵N, and D₂. The isotopic switch is typically achieved with a fast-acting four-way valve, alternating between labeled and unlabeled feeds. Analysis of the resulting transients reveals key kinetic parameters and mechanistic details.
SSITKA enables direct measurement of key kinetic parameters, including mean residence time, surface intermediate concentrations, and intrinsic rate constants. These values provide quantitative insight into active site density, reaction pathways, and the influence of supports, promoters, alloying, and deactivation mechanisms.
Ethylene, a key building block in organic synthesis, can be selectively oxidized to either acetaldehyde (using PdCl/CuCl catalysts) or ethylene oxide (EO) over silver-based catalysts. Despite decades of study, the identity of the active oxygen species (e.g., Agₓ–O vs. Ag–O–O–Ag) and the dominant reaction mechanism—whether Langmuir–Hinshelwood (L-H), Eley–Rideal (E-R), or Mars–van Krevelen (MvK)—remains under debate. To address this, Professor Israel E. Wachs and his team at Lehigh University combined in situ Raman spectroscopy with SSITKA to investigate ethylene oxidation over Ag/α-Al₂O₃ catalysts and clarify both the nature of the active sites and the operating mechanism [3].
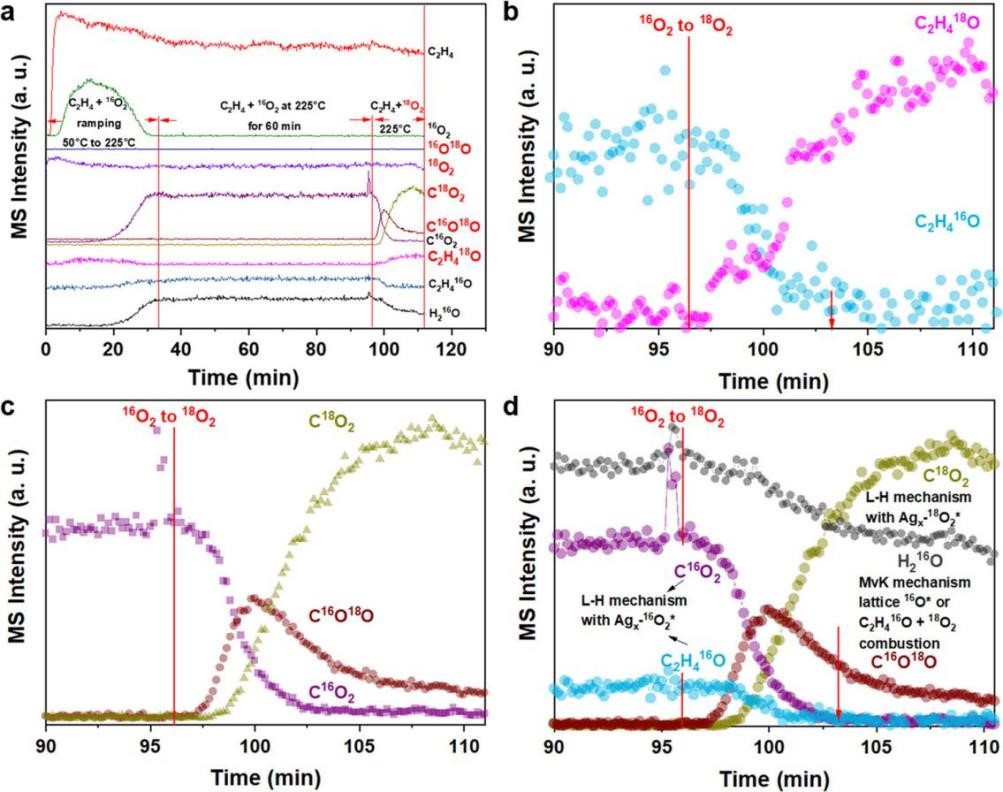
Figure 1: SSITKA Experiment for C₂= + 16O₂ → 18O₂
Figure 1 shows mass spectra from SSITKA experiments using AMI-200. After switching from C₂= + 16O₂ → 18O₂ (Figure 1b, c), EO and CO₂ signals rapidly decay to zero within ~7 minutes, confirming ethylene epoxidation follows the L-H mechanism (requiring adsorbed ethylene and Ag₄-O₂ species). Post-switch, C₂H₄16O and C16O₂ signals gradually decrease, while C16O18O rises, indicating MvK (lattice oxygen) participation in CO₂ formation. SSITKA demonstrates that ethylene epoxidation predominantly follows L-H, while complete oxidation involves both L-H and MvK mechanisms.
SSITKA in Fischer-Tropsch (F-T) Synthesis
The conversion of synthesis gas (CO + H₂) into clean fuels and value-added chemicals is a key pathway for the efficient utilization of coal. However, the complexity of the product distribution and low selectivity of the reaction pose challenges to its industrial application. In recent years, cobalt-based catalysts have gained attention for producing high-quality diesel fuels, owing to their relatively slow deactivation and favorable carbon chain growth characteristics. While catalyst additives are known to significantly influence performance, their effects on reaction kinetics remain underexplored. To address this, Professor Yang Jia’s team at the Norwegian University of Science and Technology, in collaboration with Professor Xiaoli Yang’s group at Qingdao University, modified Co-based catalysts with Rh, Ir, Sb, and Ga to evaluate their effects on Fischer–Tropsch synthesis [4]. SSITKA was used to investigate the catalysts’ intrinsic activity and surface adsorption behavior, providing deeper insight into the role of these additives in reaction kinetics.
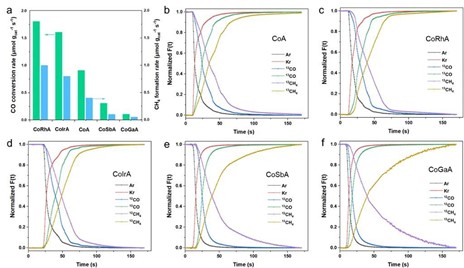
Figure 2: (a) CO conversion rate and CH4 formation rate on CoM/Al2O3 catalyst under SSITKA condition. Normalized transient curves of (b) CoA, (c) CoRhA, (d) CoIrA, (e) CoSbA, and (f) CoGaA.
SSITKA experiments involved switching from 12CO/H₂/Ar to 13CO/H₂/Ar. Normalized transient curves (Figure 2) were analyzed to derive parameters in Table 1:
Table 1: SSITKA Parameters for Co-Based
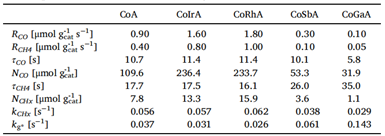
Through steady-state SSITKA parameter analysis, it is found that: The amount of NCO surface intermediates of the reactants of CoRh/Al2O3 and CoIr/Al2O3 was (236 and 234 μmol gcat-1), respectively, which was 2 times higher than that of CoA (109.6 μmol gcat-1). The reduction of CoSb/Al2O3 and CoGa/Al2O3 catalysts is 53 and 39 μmol gcat-1, respectively. These data indicate that the precious metals Ir and Rh can promote the catalyst and increase more active sites, so that more CO can be adsorbed. The quantity of intermediates on the surface of the product NCHx has the same trend. However, non-precious metal Sb and Ga auxiliaries showed the opposite trend. At the same time, the residence time of the four auxiliaries was analyzed, and it was found that the residence time of the four auxiliaries had little difference, and the precious metal had no obvious effect on the intrinsic activity of the catalyst, while the non-precious metal reduced the active site and the intrinsic active site.
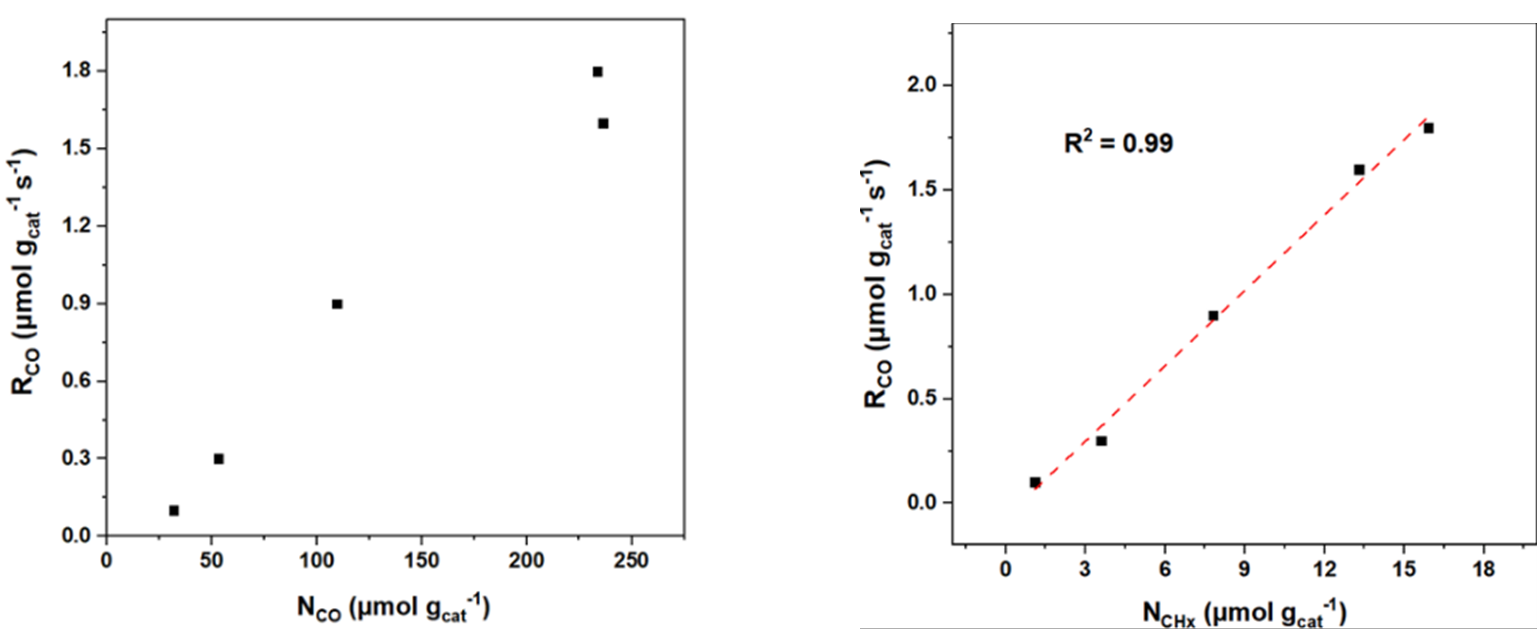
Figure 3: Relation between CO reaction rate of CoM/Al2O3 and concentration of CO and CHx intermediates
Figure 3 compares the CO reaction rate, surface intermediate concentrations, and intrinsic rate constants. The reaction rate of CO was found to be independent of NCO concentration, suggesting that CO conversion is not directly governed by NCO coverage, but rather by the surface coverage of CO and H₂. A linear relationship with NCHₓ concentration was observed, indicating that CHₓ intermediates play a key role in determining the reaction rate of CO. Figure 4 illustrates the proposed carbon chain growth pathway. The addition of Ir and Rh was found to inhibit carbon chain growth, while Sb and Ga promoted it. These additives influence the surface concentration of CHₓ species during the reaction, which in turn affects both the CO reaction rate and the carbon chain growth rate constant, ultimately altering the product distribution. Further analysis suggests that these effects stem from electronic modifications introduced by the additives. Changes in the electronic properties of the active metal lead to reduced adsorption strength, thereby shifting surface reactivity and selectivity.
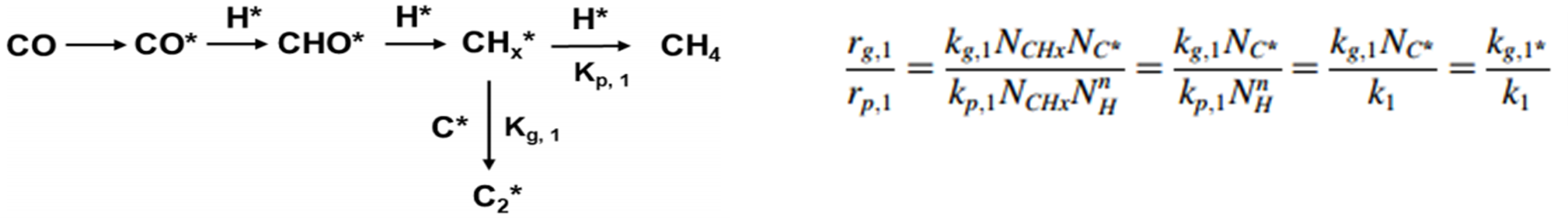
Figure 4: Reaction Pathways for CHₓ Intermediate Conversion to CH₄ and C₂+
In summary, this paper by Yang J. et al. provides kinetic insight into how different additives influence the performance of Co-based catalysts in Fischer–Tropsch synthesis. Their work identifies the key kinetic factors underlying enhanced catalytic activity and offers a foundation for further catalyst optimization.
Steady-State Isotopic Transient Kinetic Analysis (SSITKA) is a powerful technique for quantifying surface intermediates and extracting kinetic parameters under true reaction conditions. With advancements in instrumentation and analytical methods, SSITKA is increasingly combined with complementary approaches such as in situ spectroscopy, kinetic modeling, and DFT calculations to provide a more comprehensive understanding of catalytic mechanisms. Recognizing the growing need for integrated, precise, and user-friendly tools, the AMI-300SSITKA system—a dedicated platform designed to perform reliable SSITKA experiments with high temporal resolution, stable gas switching, and seamless coupling to mass spectrometry. In future articles, we will explore the underlying principles of SSITKA and demonstrate how the AMI-300SSITKA supports advanced catalyst characterization and kinetic analysis.
[1] Recent Approaches in Mechanistic and Kinetic Studies of Catalytic, Cristian Ledesma, Jia Yang, De Chen, and Anders Holmen, ACS Catal. 2014, 4, 4527−4547
[2] Li, C. Y., & Shen, S. K. (1999). Steady-state isotopic transient kinetic analysis. Progress in Chemistry, 11(2), 49–59.
[3] Tiancheng Pu, Adhika Setiawan, Revealing the Nature of Active Oxygen Species and Reaction Mechanism of Ethylene Epoxidation by Supported Ag/-Al2O3 Catalysts: ACS Catal. 2024, 14, 406−417.
[4] Xiaoli Yang, Jia Yang, Anders Holmen, Kinetic insights into the effect of promoters on Co/Al2O3 for Fischer-Tropsch synthesis, Chemical Engineering Journal 445 (2022) 136655.