Advanced Measurement Instruments
Copyright © 2025 Advanced Measurement Instruments
Abstract
This application note presents a study on the selective adsorption behavior of small molecule hydrocarbons—acetylene (C₂H₂), ethylene (C₂H₄), propane (C₃H₈), and propylene (C₃H₆)—on various metal-organic framework (MOF) materials. Using AMI’s Micro 300 for high-precision static adsorption isotherms, this work highlights the potential of MOFs in non-cryogenic, energy-efficient separation of light hydrocarbons. Although dynamic breakthrough testing was not performed in this study, AMI’s BTsorb 100 system is noted as an ideal platform for future validation under flow conditions.
Introduction
In the petrochemical industry, C₂ hydrocarbons are foundational to the production of downstream products including polymers, rubbers, and specialty chemicals. However, separating these components remains difficult due to their similar boiling points and molecular sizes. Conventional cryogenic distillation is energy-intensive and cost-prohibitive.
Recent studies have demonstrated that MOF materials—due to their tunable pore size and chemically functionalized internal surfaces—offer a promising solution for energy-efficient separation of these hydrocarbons. Examples include the SIFSIX series, known for acetylene/ethylene selectivity (FIGURE 1), and flexible frameworks like sql-SIFSIX-bpe-Zn, which undergo reversible transformations in the presence of C₂H₂ (FIGURE 2). Additionally, MIL-142A, a cross-linked Fe-MOF, has shown remarkable capacity and selectivity for C₃H₈ over CH₄ under ambient conditions (FIGURE 3).
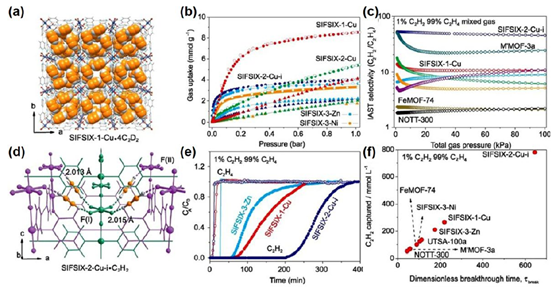
Figure 1: (a) SIFSIX-1-Cu-4C2D2 Structural Diagram and (b-f) C₂H₂ Adsorption and C₂H₂/C₂H₄ Separation Schematic
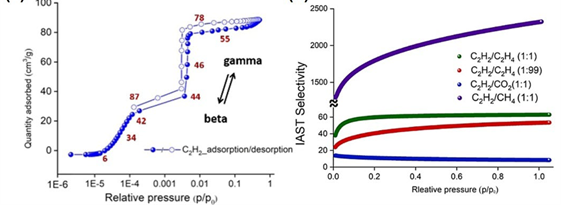
Figure 2: (Left) Adsorption Changes of C₂H₂ during the SC-SC Transition of the two-dimensional Flexible MOF Material sql-SIFSIX-bpe-Zn; (Right) C₂H₂/C₂H₄ Separation Ratio.
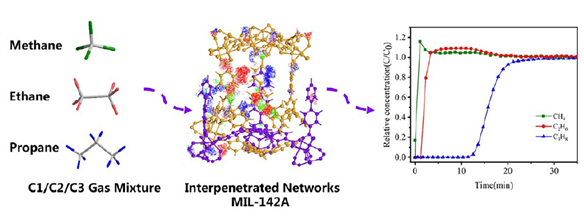
Figure 3: C1/C2/C3 Three-component Gas Separation Diagram of MIL-142A
Experimental Methods
Instruments
Conditions
Results and Discussion
C₂ Hydrocarbon Adsorption
Adsorption isotherms recorded on the AMI Micro 300 revealed a distinct difference in uptake behavior between acetylene and ethylene. For MOF-1, acetylene displayed a steep increase in adsorption between 4–6 kPa, followed by saturation (FIGURE 4). Ethylene, by contrast, showed negligible adsorption across the tested pressure range.
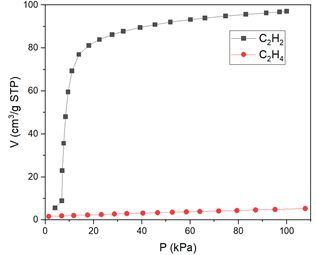
Figure 4: Adsorption Isotherm on Micro 300
These results are consistent with the known affinity of fluorinated MOFs for triple-bonded hydrocarbons, likely due to π-H interactions with exposed SiF₆²⁻ groups.
C₃ Hydrocarbon Selectivity
Further experiments evaluated the adsorption of propane and propylene on MOF-2 and MOF-3. Both materials exhibited strong uptake of propylene while showing no detectable adsorption of propane (FIGURE 5). The clear selectivity suggests that steric effects and kinetic diameter differences influence uptake behavior.
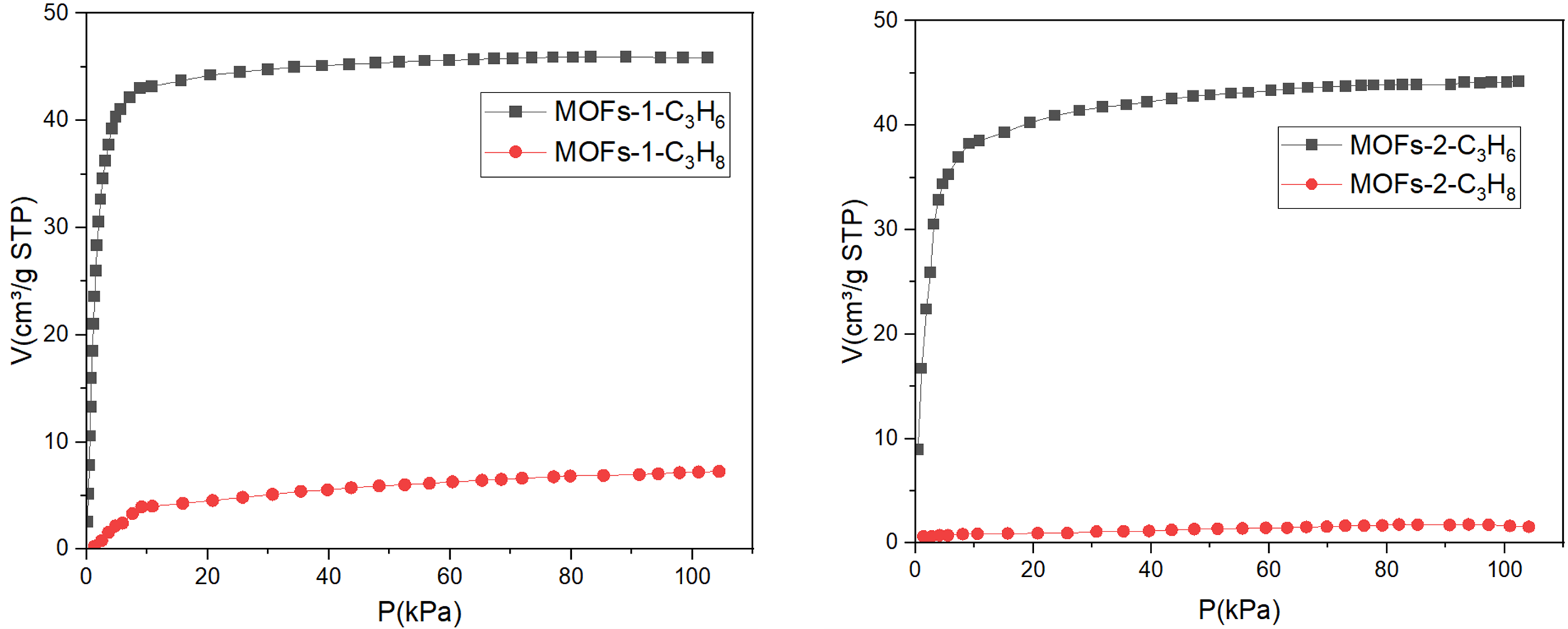
Figure 5: Adsorption Isotherm on Micro 300 of MOF 2 and 3
Note on Dynamic Testing
Although dynamic breakthrough testing was not conducted as part of this study, the AMI BTsorb 100 is designed for such evaluations and remains a valuable tool for future studies aimed at simulating industrial gas separation scenarios.
Applications
These findings indicate that:
By pairing AMI’s Micro 300 for equilibrium data and the BTsorb 100 for future dynamic testing, researchers can comprehensively assess adsorbent materials for industrial gas separation applications.
Conclusion
This study underscores the promise of MOF-based adsorbents for targeted separation of light hydrocarbons at ambient conditions. While this work focused on static adsorption behavior, AMI’s suite of instruments—especially the Micro 300 and BTsorb 100—provides a scalable, versatile platform for future full-cycle evaluation from material screening to process development.