Advanced Measurement Instruments
Copyright © 2025 Advanced Measurement Instruments
Reducing atmospheric CO₂ concentrations remains one of the most pressing challenges in climate science and industrial decarbonization. Carbon Capture and Storage (CCS) has emerged as one of the most effective approaches for mitigating CO₂ emissions, with several core technologies under active development: membrane separation, solid adsorption, and liquid absorption.
Membrane separation relies on the selective permeation of gas molecules through materials such as inorganic or organic polymer membranes. Inorganic membranes (e.g., molecular sieves, porous ceramics) offer excellent chemical and thermal stability, but tend to have higher material and processing costs. Organic polymer membranes, while more economical, are limited by thermal
sensitivity, which restricts their use in high-temperature CO₂ capture scenarios.
Solid adsorption captures CO₂ through interactions between gas molecules and the surface of porous materials. Two mechanisms are possible:
Liquid absorption can also be divided into physical and chemical categories.
While chemical solvents offer high capacity and fast absorption rates, several legacy solutions (e.g., KOH and ammonia) face challenges related to equipment corrosion, volatility, and handling safety. Today, amine-based absorbents are the most widely used due to their favorable balance of reactivity, efficiency, and scalability in industrial CO₂ capture.
Organic amines, commonly used in chemical CO₂ absorption systems, contain hydroxyl (–OH) and amino (–NH₂, –NHR, –NR₂) functional groups. The hydroxyl group improves water solubility, while the amino group increases the solution’s pH, enhancing alkalinity and CO₂ absorption potential.
The fundamental mechanism is an acid-base neutralization reaction, in which the weakly acidic CO₂ reacts with basic amines to form a water-soluble salt. This reaction is temperature-dependent and reversible:
Amine Classification and Reactivity
Organic amines are classified by the number of hydrogen atoms substituted on the nitrogen:
The binding strength of CO₂ with these amines generally follows the order:
Primary > Secondary > Tertiary
Primary and Secondary Amines: Carbamate Formation
The widely accepted zwitterion mechanism (Caplow, Danckwerts) describes a two-step reaction:
Here, the zwitterion reacts with a base (e.g., amine, OH⁻, or H₂O) to form a carbamate. The strong C–N bond in the carbamate makes the product highly stable, but also leads to:
Maximum loading for primary/secondary amines is typically 0.5 mol CO₂ per 1 mol amine.
Tertiary Amines: Bicarbonate Formation
Unlike primary and secondary amines, tertiary amines lack reactive hydrogen atoms and do not form carbamates. Instead, they enhance CO₂ hydration and facilitate bicarbonate formation:
This route results in:
2.1 Mass Transfer Mechanism of CO₂ Absorption
The mass transfer process involved in CO₂ absorption is commonly described using the two-film theory, first proposed by Whitman and Lewis. This model divides the gas–liquid interface into five distinct regions (see Figure 1):
The mass transfer process involved in CO₂ absorption is commonly described using the two-film theory, first proposed by Whitman and Lewis. This model divides the gas–liquid interface into five distinct regions (see Figure 1):
In the bulk gas and liquid phases, turbulence is typically high, ensuring uniform composition. However, as the gas and liquid phases approach the interface, they pass through their respective stagnant film layers, where molecular diffusion becomes the dominant transport mechanism.
The CO₂ absorption sequence follows these steps:
While the two-film theory is widely used and provides a useful conceptual framework, it does have limitations. In systems with free interfaces or high turbulence, the interface becomes unstable and continuously disrupted. Under these conditions, the assumption of two steady, well-defined stagnant films on either side of the interface becomes less accurate. In such cases, convective mixing and interfacial renewal models may offer better descriptions of the actual transport dynamics.
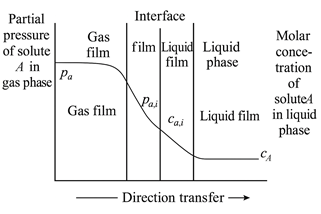
Figure 1 Schematic Diagram of the Two-Film Theory
2.2 Performance and Limitations of MEA-Based CO₂ Absorption
Monoethanolamine (MEA) is one of the most widely used absorbents for CO₂ capture. As a primary amine, MEA exhibits strong basicity and a low molecular weight, resulting in:
Due to these advantages, MEA-based absorption systems are commercially established and extensively deployed in both industrial and pilot-scale applications.
However, MEA is not without drawbacks. It has a high heat of reaction with CO₂, which translates to greater energy consumption during regeneration. MEA also tends to form stable amine carbonates and is prone to degradation when exposed to CO, sulfur compounds, or oxygen-containing gases, contributing to solvent loss and reduced operational efficiency.
Studies have shown that increasing MEA concentration improves CO₂ removal efficiency:
The improved performance is attributed to both increased vapor pressure and reaction enthalpy, which raise the temperature of the absorption solution. However, higher concentrations come with trade-offs:
For these reasons, MEA is most commonly used at ~30 wt% concentration, where performance and system stability are reasonably balanced. At higher concentrations, corrosion inhibitors are typically required.
Current research efforts are focused on:
2.3 Enhancing CO₂ Capture with Mixed Amine Solutions
To further improve CO₂ absorption efficiency and reduce energy consumption, blended alkanolamine solutions have become a key focus in solvent development. This strategy combines amines with high absorption capacity but slower kinetics with those offering faster reaction rates but lower capacity, aiming to strike an optimal balance between performance and regeneration efficiency.
By carefully selecting and mixing complementary amines, it is possible to enhance both the absorption and desorption characteristics of the solvent system. One promising approach involves blending monoethanolamine (MEA) with piperazine (PZ).
Research by Zhang Yaping [10] demonstrated that adding PZ to MEA significantly improves overall performance:
At a total amine concentration of 2 mol/L, the MEA–PZ blend achieved a saturated CO₂ absorption capacity of 206.1 mL/L, with a desorption efficiency of 86.38%. These findings highlight the potential of mixed amine systems to outperform single-component solvents in industrial carbon capture applications.
To further optimize CO₂ capture performance, recent research has focused on composite alkanolamine systems—blends that combine the strengths of individual amines to improve overall absorption capacity, regeneration efficiency, and cycling performance. These mixed-solvent systems aim to balance fast kinetics, high capacity, and low energy consumption.
Key Findings from Recent Studies
These studies demonstrate that composite amine systems can significantly improve CO₂ capture by combining complementary properties—such as fast kinetics, high loading capacity, and reduced regeneration energy. Mixed alkanolamine formulations represent a promising path forward in the design of next-generation absorbents for industrial-scale carbon capture.
In addition to modifying amine formulations through blending, recent research has explored the use of catalysts and nanoparticles to further enhance CO₂ absorption performance, improve regeneration efficiency, and reduce overall energy consumption.
Catalyst-Enhanced Amine Systems
Ali Saleh Bairq et al. [17] reported that incorporating SO₄²⁻/ZrO₂/SiO₂ into monoethanolamine (MEA) resulted in significant improvements:
These results highlight the potential of heterogeneous acid-base catalysts to promote the reversibility of CO₂ absorption reactions in amine systems.
Nanoparticle-Modified Absorbents
Nanoparticles have also emerged as effective additives, enhancing mass transfer, absorption rate, and desorption efficiency. Li Wenya et al. [18] studied the impact of TiO₂ and graphene oxide (GO) nanoparticles on diethylaminoethanol (DEEA) and N-methyldiethanolamine (MDEA) systems.
Key results compared to MEA without nanoparticles include:
| System | Absorption Capacity | Absorption Rate | Desorption Capacity | |
|
9.86% | 33.43% | 10.72% | |
| 30% MDEA + 0.05% 40 nm TiO₂ | 11.26% | 49.72% | 9.19% | |
| 30% MDEA + 0.06% GO | 13.8% | 39.23% | 26.66% |
These nano-enhanced solvents demonstrated significant improvements in both absorption and regeneration metrics, offering a path to more efficient CO₂ capture processes.
Effect of Nanoparticle Size
Jiang et al. [19] compared the performance of 10 nm and 20 nm TiO₂ nanoparticles in MEA systems. While smaller particles offer higher surface activity, they are more prone to agglomeration, which can reduce effectiveness. In contrast, larger particles (20 nm) were found to more effectively disturb the mass transfer boundary layer, enhancing local mixing, concentration gradients, and overall mass transfer performance.
Incorporating catalysts or engineered nanoparticles into alkanolamine solutions is a promising direction for improving the kinetics, capacity, and energy efficiency of CO₂ capture systems. These additive-based approaches represent a valuable area of future research for next-generation absorbents.
3.0 Experimental Method
To study the CO₂ adsorption behavior of monoethanolamine (MEA), we conducted a series of experiments using the BTSorb 100 (formally MIX100) breakthrough curve and mass transfer analyzer on a commercially available MEA solution.
Test Procedure
Experimental Conditions - Details of the test setup are summarized in Table 1 below.
Table 1 Experimental Conditions for Ethanolamine
| Sample Volume (ml) | N₂ Flow Rate (ml/min) | CO₂ Flow Rate (ml/min) | Temperature (°C) | Pressure (bar) |
| 10-1 | 185 | 15 | 40 | 1 |
| 10-1 (Re-adsorption) | 185 | 15 | 40 | 1 |
| 10-2 | 185 | 15 | 40 | 1 |
As shown in Figure 2, the breakthrough curves from the 10-1 and 10-2 tests are nearly identical, demonstrating excellent repeatability of the experimental setup. However, the 10-1 (re-adsorption) curve exhibits a slightly shorter breakthrough time, indicating a marginal decrease in adsorption capacity following the regeneration cycle.
This result suggests that while MEA retains good performance after regeneration, some loss of adsorption efficiency may occur, likely due to incomplete solvent recovery or minor thermal degradation during the desorption process.
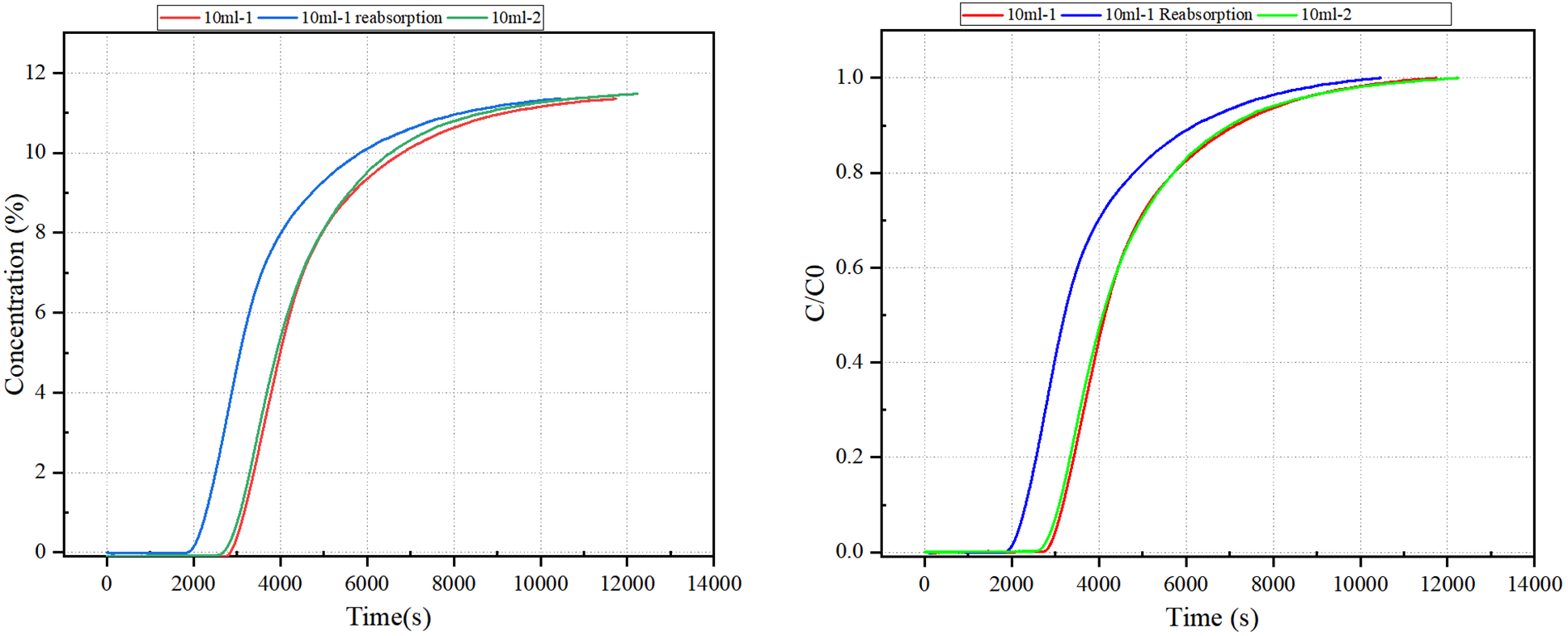
Figure 2 Breakthrough Curve of Ethanolamine MEA
Based on the calculated results presented in Table 2, the measured CO₂ adsorption capacities for the two 10 mL monoethanolamine (MEA) samples were 0.4875 mol/mol and 0.4822 mol/mol, respectively. These values are consistent with the commonly reported commercial MEA capacity of approximately 0.5 mol CO₂ per mol amine, validating the reliability of the test conditions and measurement approach.
Following desorption and re-adsorption (Test 10-1), the measured adsorption capacity decreased to 0.3875 mol/mol, indicating a notable decline in performance after regeneration. This reduction may be attributed to partial thermal degradation of MEA or incomplete recovery of active absorption sites during the desorption cycle.
Table 2 Calculated Adsorption Capacity Results for Ethanolamine MEA
| Sample Name | Adsorption Capacity mol(CO₂)/mol(MEA) |
| 10ml-1 | 0.4875 |
| 10ml-1 (Re-adsorption | 0.3875 |
| 10ml-2 | 0.4822 |
4.0 Conclusions
This study demonstrates the viability of using monoethanolamine (MEA) as a chemical absorbent for CO₂ capture under ambient conditions. Through a combination of theoretical review and experimental validation using the BTSorb 100 (formally MIX100) breakthrough analyzer, MEA was shown to achieve CO₂ adsorption capacities consistent with commercial expectations (~0.5 mol/mol). While regeneration was successful, a decline in adsorption performance after the desorption cycle suggests some loss in efficiency, likely due to thermal degradation or incomplete solvent recovery.
The data confirms that MEA remains a strong candidate for CO₂ absorption systems, especially when optimized with temperature control and proper regeneration protocols. Future improvements may be achieved through blending with secondary or tertiary amines, use of corrosion inhibitors, or incorporation of nanomaterials and catalysts to reduce energy consumption and enhance cycling performance. These directions represent promising opportunities for scaling liquid-phase CO₂ capture in industrial applications.
5.0 References
[1] Wang J., Huang L., Yang R., et al. Recent advances in solid sorbents for CO₂ capture and new development trends. Energy & Environmental Science, 2014, 7: 3478–3518.
[2] Venna S.R., Carreon M.A. Highly permeable zeolite imidazolate framework-8 membranes for CO₂/CH₄ separation. Journal of the American Chemical Society, 2010, 132(1): 76–78.
[3] Huang Yuhui. Research on the Degradation of Mixed Amine Absorbents for Flue Gas CO₂ Chemical Absorption Technology [D]. Hangzhou: Zhejiang University, 2021.
[4] Wang Dong, et al. Research progress on solid and liquid adsorbents for carbon dioxide capture.