Advanced Measurement Instruments
Copyright © 2025 Advanced Measurement Instruments
Background
Organic compounds frequently exist in multiple polymorphic forms, each exhibiting distinct physical properties such as solubility, melting point, or heat of fusion. Polymorphism plays a significant role in pharmaceutical development, particularly for poorly soluble drugs.
The following well-known thermodynamic equation relates solubility (X) to heat of fusion (ΔHf), melting point (Tm), and heat capacity change (ΔCp) [1]:
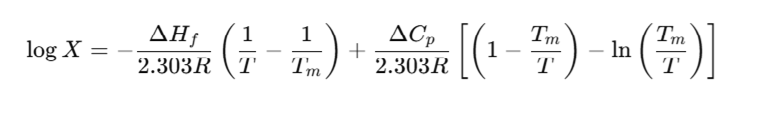
By applying this equation to two anhydrous polymorphs of carbamazepine (Forms I and III), and solving the equations simultaneously, one can derive the ratio of their solubilities. This theoretical approach, when combined with experimental calorimetric data, yields a calculated transition temperature that agrees closely—within 2°C—with the value estimated from experimental solubility data [2].
Experimental Thermodynamic Parameters
Forms I and III of carbamazepine show a melting point difference of 15°C:
These values were used to calculate the solubility ratio profile, as shown in Figure 1 (closed circles). Theoretical curves in the same figure demonstrate the impact of varying the ΔHf difference (4.5, 6.0, 7.5, and 9.0 kJ/mol) between the two forms.
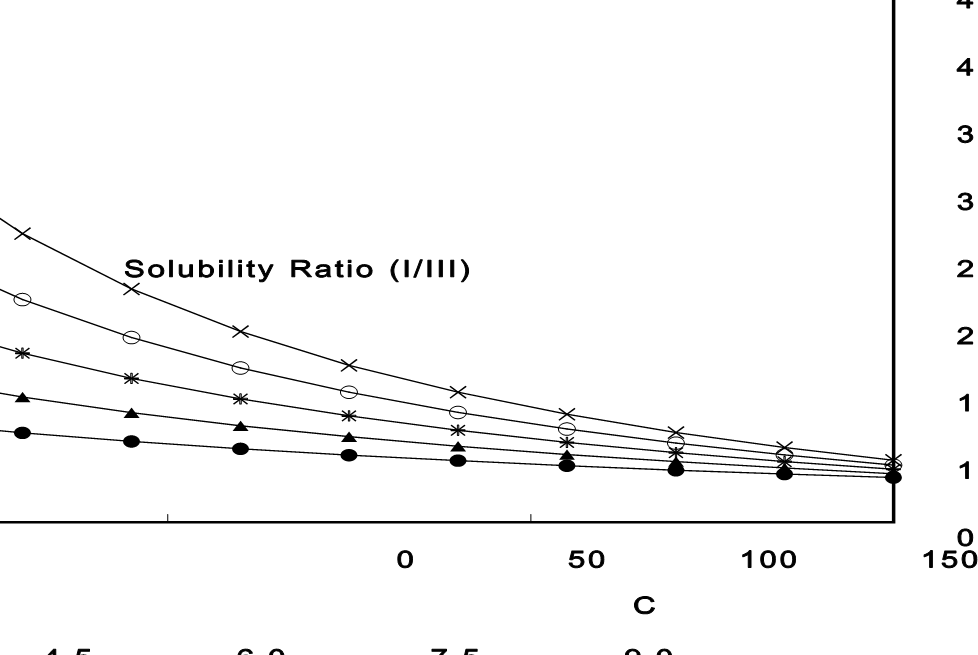
Figure 1
These simulations show that even if the ΔHf difference had been as high as 9.0 kJ/mol, Form I would have been only about three times more soluble than Form III at room temperature—demonstrating the modest influence of heat of fusion differences on solubility ratio.
Effect of Melting Point Differences on Solubility
To further evaluate the influence of melting point, the actual ΔHf values of the two polymorphs were held constant, while the melting point difference was varied across a broad range—up to 37.5°C.
As shown in Figure 2, these simulations again indicate a modest effect: even with large melting point differences, Form I would have been less than twice as soluble as Form III.
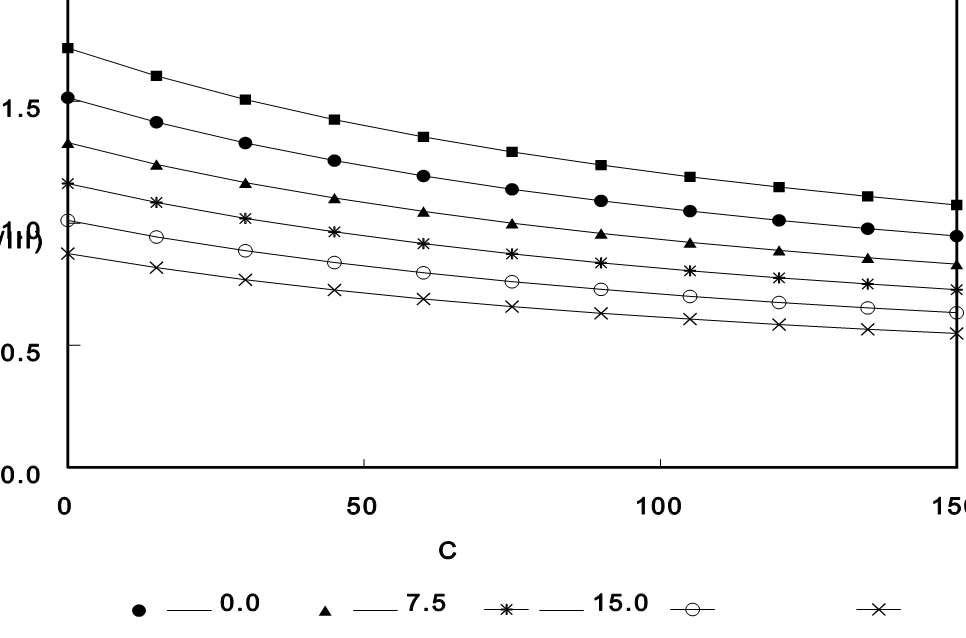
Figure 2
Discussion and Implications
The theoretical treatment supports a key observation from previous experimental studies: polymorphs rarely show dramatically different solubilities [2]. This finding has practical consequences for pharmaceutical formulation:
Accurate detection of melting points and heats of fusion is critical to these analyses. The DSC 600 by AMI offers high-resolution thermal detection, exceptional baseline stability, and precise integration tools for quantifying enthalpic transitions—making it ideal for evaluating polymorphic behavior and supporting solubility modeling in early formulation work.
References
[1] David J. W. Grant and Takeru Higuchi in Techniques of Chemistry, Volume XXI, Solubility Behavior of Organic Compounds; J. Wiley & Sons: New York, 1990.
[2] Behme, R. J.; Brooke, D. J. Pharm. Sci. 1991, 80, 986–990.