Advanced Measurement Instruments
Copyright © 2025 Advanced Measurement Instruments
Research Background
Heterogeneous catalytic processes are extremely complex surface physicochemical processes. The main participants in these processes are catalysts and reactant molecules, primarily involving the cyclic repetition of elementary steps, including diffusion, chemical adsorption, surface reaction, desorption, and reverse diffusion. The most critical steps are adsorption and surface reaction.
Therefore, to elucidate the intrinsic role of a catalyst in a catalytic process and the interaction mechanism between reactant molecules and the catalyst, it is necessary to investigate the catalyst's intrinsic structure (e.g., specific surface area and pore structure), adsorption properties (e.g., structure of adsorption centers, energy state distribution, adsorption states of molecules on adsorption centers), and catalytic properties (e.g., nature of active catalytic sites, metal dispersion). Further studies should also include mass transfer processes, reaction mechanisms, long-term stability, and pilot-scale evaluation to comprehensively assess catalyst effectiveness.
Solutions
2.1 Pore Structure
Heterogeneous catalytic reactions occur on the surface of solid catalysts. To maximize reaction activity per unit volume or weight, most catalysts are designed with porous structures to increase surface area. The porous structure and pore size distribution directly influence diffusion and mass transfer, which in turn affect catalytic activity and selectivity.
Zeolite catalysts are a class of microporous crystalline materials with uniform pore structures and extremely high surface areas. Figure 1 demonstrates nitrogen adsorption-desorption curves. In the low-pressure region, nitrogen adsorption sharply increases due to micropore filling. The HK pore size distribution reveals the most probable diameter is 0.57 nm, and the BET surface area is calculated as 675 m²/g.
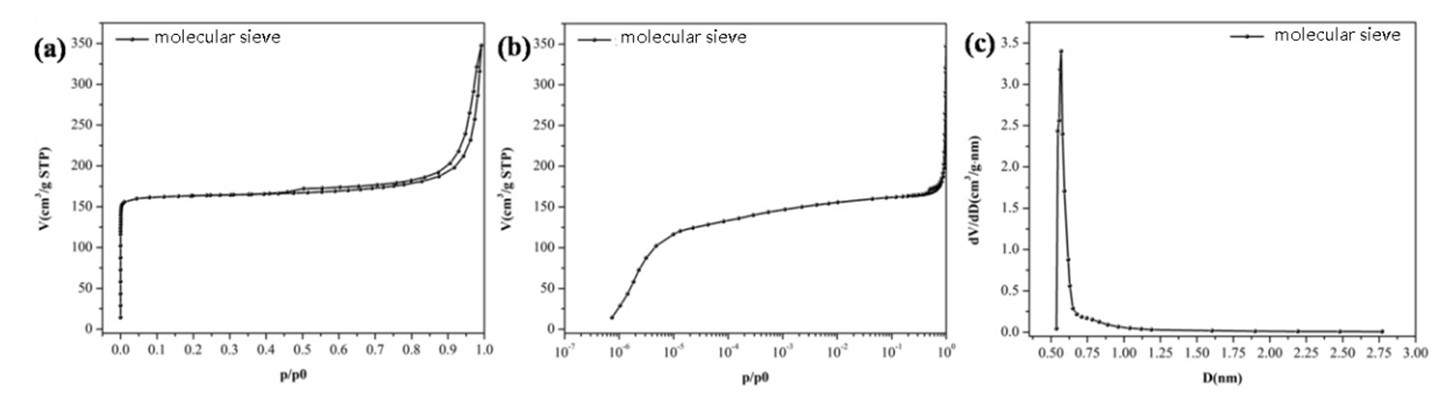
FIGURE 1: Zeolite N₂ Adsorption-Desorption Isotherms (A) Linear Scale, (B) Logarithmic Scale, (C) HK Micropore Size Distribution
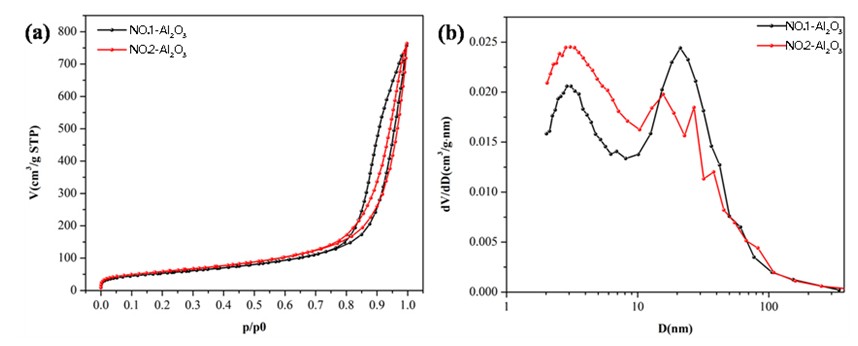
ctivated alumina is another widely used catalyst support. It shows excellent surface acidity and thermal stability. Figure 2 shows adsorption curves of two alumina samples with surface areas of 192.32 m²/g and 210.81 m²/g. BJH analysis indicates pore size peaks at 3 nm and 21 nm.
Nickel-loaded cerium dioxide (CeO₂) catalysts are notable for their redox cycling and oxygen storage. Figure 3 shows how increasing Ni loading reduces surface area (from 15.25 to 7.59 m²/g) and pore volume, due to Ni occupying surface sites.
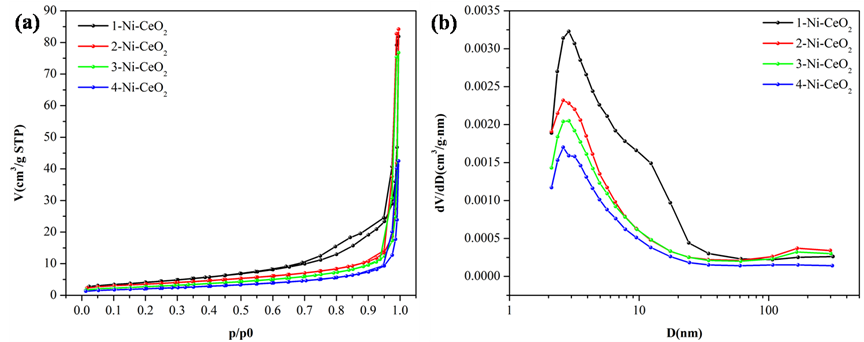
FIGURE 3: (a) Ni@CeO₂ N₂ Adsorption-Desorption Isotherms, (b) BJH Pore Size Distribution
2.2 Active Centers
The intrinsic active sites of catalysts are key to their reactivity. These are best characterized dynamically under operating conditions. AMI systems apply temperature-programmed techniques (TPx series) for this purpose.
Temperature-Programmed Reduction (TPR)
TPR measures reducibility and interactions of active metal oxides with supports. Figure 4(a) shows a metal-supported alumina catalyst with a single strong reduction peak at 234°C and hydrogen consumption of 9680 µmol/g. Figure 4(b) shows three distinct peaks for Mn₂O₃ → Mn₃O₄ → MnO → Mn transformations. Figure 4(c) compares different Ni loadings on CeO₂, showing increasing reduction temperatures and decreasing hydrogen consumption as Ni loading increases.
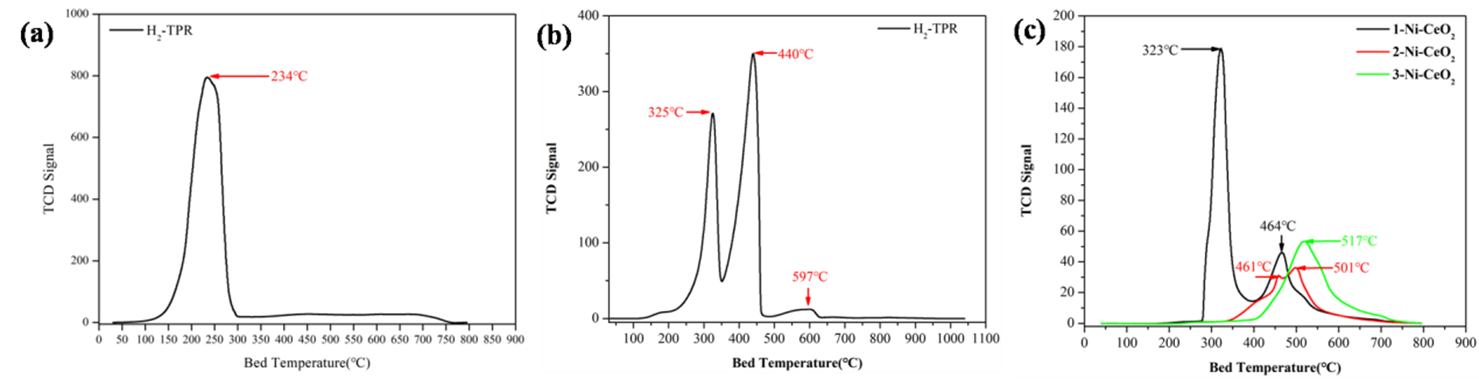
FIGURE 4: H₂-TPR (a) Alumina-supported Catalyst, (b) Mn₂O₃-based Catalyst, (c) Ni@CeO₂ Catalyst Temperature-Programmed Oxidation (TPO)
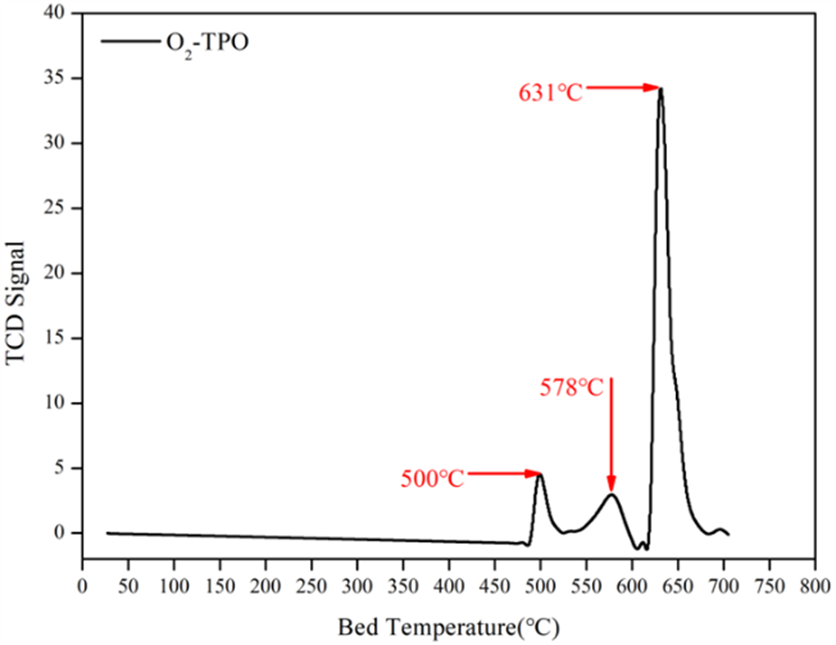
TPO is used to evaluate coke deposition and regeneration conditions. Figure 5 shows TPO data for a Cr₂O₃ catalyst after reaction, with three peaks at 500°C, 578°C, and 631°C. The high-temperature coke species dominate, indicating a need for high-temperature regeneration.
Conclusion
Comprehensive catalyst characterization requires more than surface-level insight. From understanding pore architecture to quantifying redox behavior and coke formation, each technique provides a piece of the puzzle.
The integration of N₂ physisorption, TPR/TPO/TPSR, and pulsed chemisorption is essential for evaluating both performance and durability under realistic reaction conditions.
AMI’s chemisorption and physisorption instrument platforms enable researchers to conduct these analyses with precision and flexibility, combining automated gas handling, programmable thermal profiles, and real-time detection in a unified workflow.
Whether developing next-generation catalysts or optimizing industrial formulations, AMI provides the tools needed to accelerate R&D and ensure reliable, reproducible results.
With proven solutions for academia, government labs, and industry, AMI continues to support catalyst development from lab-scale discovery to pilot-scale deployment.