Advanced Measurement Instruments
Copyright © 2025 Advanced Measurement Instruments
Thermal analysis techniques are widely applied in pharmaceutical research for evaluating polymorphism, phase transitions, hydration states, decomposition behavior, purity, compatibility, and stability. Among these techniques, Differential Scanning Calorimetry (DSC) and Thermogravimetric Analysis (TGA) are most frequently used and are officially recognized in many pharmacopeias for drug quality control.
Differential Scanning Calorimetry (DSC) measures heat flow associated with thermal events during a controlled temperature program. This includes endothermic and exothermic transitions such as melting, glass transitions, recrystallization, and polymorphic transformations. In the pharmaceutical field, DSC is often used to determine melting points, glass transitions, drug purity, polymorphs, and compatibility between active pharmaceutical ingredients (APIs) and excipients.
Thermogravimetric Analysis (TGA) records changes in a sample’s mass as a function of temperature under a defined atmosphere. TGA is valuable for characterizing the presence of adsorbed water, crystallization solvents, thermal stability, and decomposition behavior. For hydrates and solvates, TGA can differentiate between loosely bound and tightly bound solvent molecules by observing weight loss patterns across different temperature ranges.
Revefenacin, a long-acting muscarinic antagonist (LAMA), is used as a maintenance therapy for chronic obstructive pulmonary disease (COPD). It improves lung function, alleviates symptoms, and slows disease progression. Revefenacin exhibits polymorphism, with multiple crystalline forms (I–IV) reported. Only Form III is currently used in commercial formulations due to better processability. However, challenges such as solvate formation and solvent residue during crystallization necessitate continued development of more stable forms. For example, Revefenacin trihydrate Form A has demonstrated improved chemical stability, higher polymorphic purity, and enhanced solubility.
Thermal analysis was conducted using the AMI DSC 600 and AMI TGA 1000 systems.
DSC Characterization
Powdered Revefenacin samples were placed in aluminum crucibles and analyzed under a nitrogen purge (50 mL/min). Two heating rates—5 K/min and 10 K/min—were evaluated over a temperature range from 5 °C to 200 °C. The impact of crucible type (sealed vs. unsealed) and heating rate on the detection of polymorphic forms was investigated.
TGA Characterization
Powdered samples were placed in platinum crucibles and analyzed under a nitrogen atmosphere (50 mL/min). The temperature was ramped from room temperature to 300 °C at a rate of 5 °C/min.
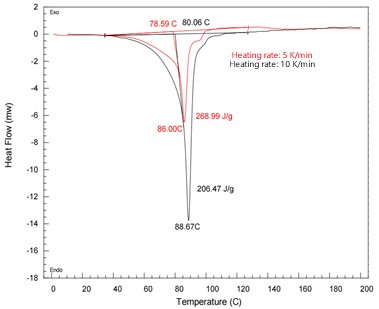
Figure 1: DSC curves of Revefenacin at different heating rates
At higher heating rates (10 K/min), the melting peaks are larger and sharper due to faster energy input. At 5 K/min, a secondary melting peak becomes visible, indicating the presence of another polymorph. This suggests that slower heating rates may improve the resolution of closely spaced transitions and help distinguish between multiple crystalline forms. Appropriate heating rate selection is therefore essential for accurate polymorph identification.

Figure 2: DSC curves of Revefenacin using sealed vs. solid crucibles
The melting behavior is influenced by the crucible type. In sealed crucibles, a clean melting peak is observed. In open (solid) crucibles, adsorbed and crystallization water evaporates during initial heating, leading to a broader melting profile beginning near 30 °C. This early peak is attributed to moisture loss rather than actual melting.
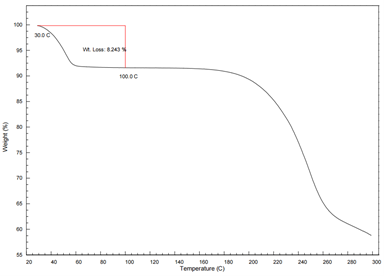
The TGA curve supports the DSC findings by showing a stepwise mass loss, corresponding to the removal of adsorbed and crystallization water. A total weight loss of 8.243% was recorded. From the weight loss between plateaus, the molar ratio of crystallization water can be calculated, confirming the hydrate content and its thermal release characteristics.
Conclusion
The AMI DSC 600 and AMI TGA 1000 provided complementary thermal and gravimetric data critical for evaluating Revefenacin’s polymorphic forms and hydration behavior.
These methods play a vital role in the development and quality assurance of pharmaceutical compounds where polymorphic stability, solvate formation, and residual solvent content are major concerns.