Advanced Measurement Instruments
Copyright © 2025 Advanced Measurement Instruments
Abstract
Hydrogen's high gravimetric energy density (120 MJ/kg) positions it as a critical energy carrier for the transition to clean energy systems. However, its extremely low density at ambient conditions significantly limits its volumetric energy density, complicating storage and transportation. Among various hydrogen storage strategies, solid-state hydrogen storage has emerged as the most promising due to its safety, efficiency, and volumetric advantages. This application note presents a comprehensive analysis of hydrogen storage materials—including Mg-based hydrides, rare earth alloys, carbon-supported systems, and MOFs—evaluated using AMI advanced sorption instrumentation.
Introduction
The volumetric energy density of hydrogen is limited by its low density at ambient conditions—0.0824 kg/m³ compared to 1.184 kg/m³ for air. Methane and gasoline have volumetric energy densities of approximately 0.04 MJ/L and 32 MJ/L, respectively. Hydrogen’s flammability, diffusivity, and explosion risks further challenge its storage [1].
Three primary hydrogen storage methods are commonly used:
Solid-state hydrogen storage stands out for safety, energy density, and moderate operating conditions, relying on physical adsorption or reversible chemical bonding. The development of high-performance materials is now central to advancing this technology.
Material Classes and Storage Mechanisms
Magnesium hydride (MgH₂) offers a theoretical hydrogen capacity of ~7.6 wt% and excellent stability under ambient conditions. However, its high desorption enthalpy (ΔH = 76 kJ/mol) and poor kinetics limit practical use. Agglomeration during cycling also reduces reversibility.
Approaches to Improve MgH₂ Performance:
Alloying Mg with elements like Ni, La, Ce, or Pr forms metastable phases, reducing reaction temperatures and improving kinetics.
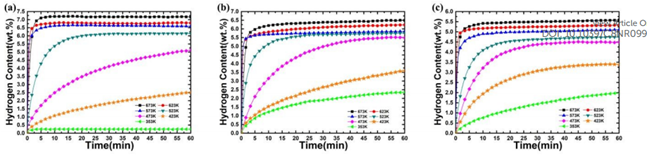
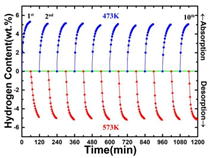
Figure: Adsorption Kinetics at Different Temperatures & Cyclic Adsorption Performance
Carbon materials improve dispersion, prevent agglomeration, and enhance hydrogen kinetics via electron transfer and surface defects.
LaNi₅ offers:
Zhu et al. [7] and Liu et al. [8] showed LaNi₅₋ₓCoₓ alloys maintain structure and capacity over 1000 cycles. Substituting La with Pr, Ce, or Gd improves equilibrium pressure and kinetics. Neto et al. [9] demonstrated improved absorption kinetics in PEI-LaNi₅ composite films at 40°C/20 bar.
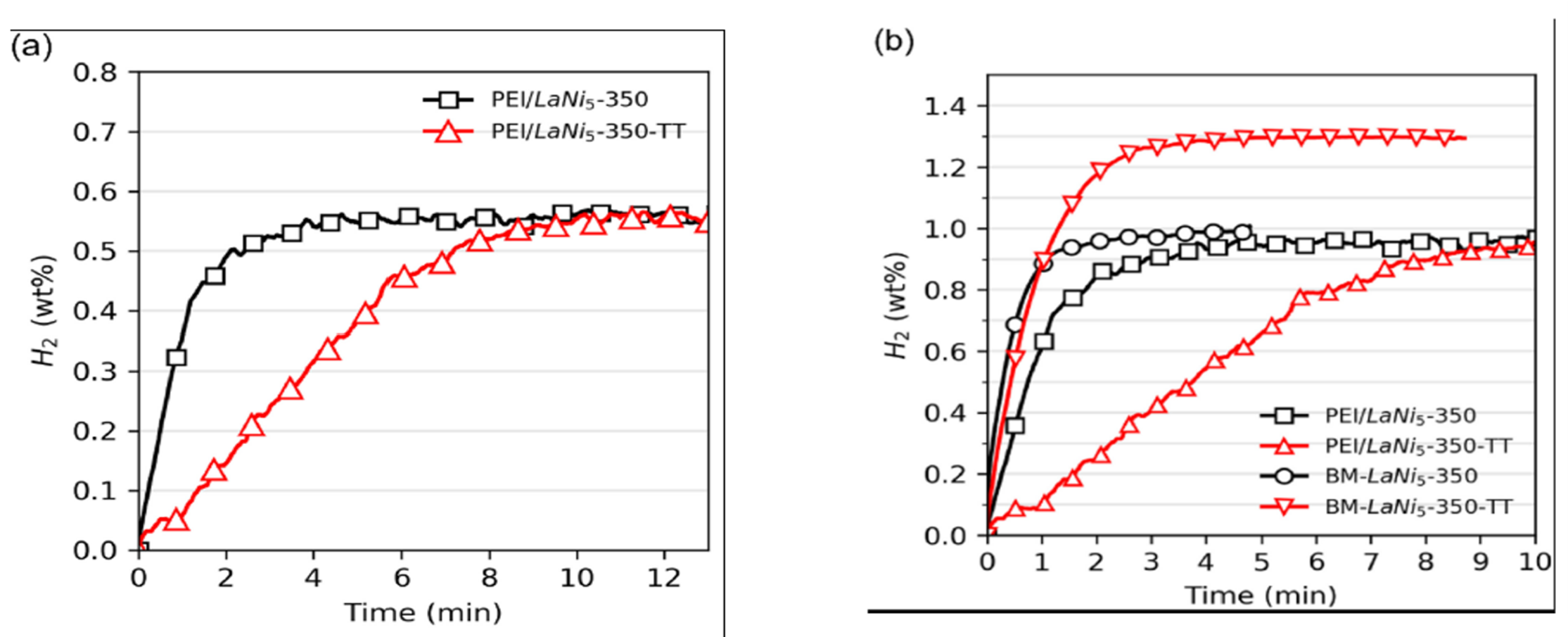
Figure: Effects of Different Annealing Conditions on Hydrogen Absorption Kinetics
MOFs combine high porosity, surface area, and tunable pore chemistry for physisorption-based H₂ storage.
Experimental Evaluation Using AMI
Using the RuboSorp MPA, AMI’s high-pressure volumetric gas sorption analyzer, LaNi₅ was tested at room temperature under pressures up to 3 MPa. Results showed rapid hydrogen uptake at low pressures (to 1.35 wt%), saturating at six hydrogen atoms per unit cell—consistent with theoretical expectations. An observable hysteresis loop indicated structural changes in LaNi₅ during hydrogen cycling.
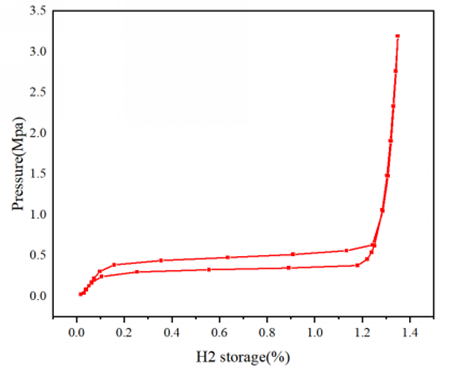
Figure: Pressure vs. hydrogen uptake curve for LaNi₅ using RuboSorp MPA
Using the RuboSorp MSB, a high-precision magnetic suspension balance system, real-time weight changes during hydrogen adsorption were recorded. The MSB provides higher accuracy than traditional volumetric systems and enables visualization of subtle structural changes through its unique high-resolution volume acquisition capabilities.

Figure: Gravimetric hydrogen uptake curve recorded by RuboSorp MSB
Hydrogen desorption kinetics of commercial MgH₂ were evaluated using the AMI-300 and AMI-400TPx. TCD-based thermal desorption testing revealed that faster heating rates increased the hydrogen evolution temperature (421–435°C) and slightly reduced total hydrogen release (7.12 → 6.98 wt%). These results illustrate the importance of thermal uniformity and diffusion efficiency during dynamic desorption.
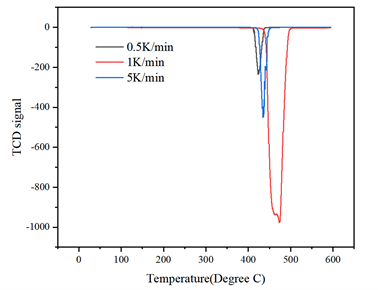
Figure: Desorption peaks of MgH₂ at varying heating rates
Conclusion
Solid-state hydrogen storage materials—especially Mg-based hydrides, LaNi₅ alloys, and MOFs—offer a viable route to safe, efficient hydrogen storage. Key factors such as thermodynamic stability, kinetic barriers, nanostructuring, and composite design strongly affect performance. Using AMI’s RuboSorp MPA, RuboSorp MSB, and the AMI-300 and AMI-400TPx systems enables precise evaluation of these effects. These instruments provide a comprehensive toolkit to support the development and commercialization of next-generation hydrogen storage materials.
References
[1] Preuster, P., Alexander, A., Wasserscheid, P. Hydrogen storage technologies for future energy systems. Annu Rev Chem Biomol Eng., 8 (2017), 445–475.
[2] Zhang, X., Liu, Y., Ren, Z., et al. Realizing 6.7wt% reversible storage of hydrogen at ambient temperature with non-confined ultrafine magnesium hydrides. Energy & Environmental Science, 14(4): 2302–2313, 2021.
[3] Samantaraya, S. S., Anees, P., Parambath, V. B., Ramaprabhu, S. Graphene Supported MgNi Alloy Nanocomposite as a Room Temperature Hydrogen Storage Material – Experiments and Theoretical Insights. Acta Materialia, 215: 117040, 2021.
[4] Ouyang, L. Z., Qin, F. X., Zhu, M. The hydrogen storage behavior of Mg₃La and Mg₃LaNi₀₋₁. Scripta Materialia, 55(12): 1075–1078, 2006.
[5] Reyhani, A., Mortazavi, S. Z., Mirershadi, S., Golikand, A. N., Moshfegh, A. Z. H2 adsorption mechanism in Mg modified multi-walled carbon nanotubes for hydrogen storage. [J]International Journal of Hydrogen Energy, 2012, 37(5): 1919-1926.
[6] Zhu, Z., Zhu, S., Lu, H., et al. Stability of LaNi5-x Cox alloys cycled in hydrogen—Part 1. Evolution in gaseous hydrogen storage performance. [J] International Journal of Hydrogen Energy, 2019, 44(29): 15159-15172.
[7] Liu, J., Zhu, S., Zheng, Z., et al. Long-term hydrogen absorption/desorption properties and structural changes of LaNi4 Co alloy with double desorption plateaus. [J] Journal of Alloys and Compounds, 2019, 778: 681-690.
[8] Neto, G. R. de A., Beatrice, C. A. G., Leiva, D. R., Pessan, L. A. Polyetherimide-LaNi5 composite films for hydrogen storage applications. [J] International Journal of Hydrogen Energy, 2021, 46(23).
[9] Musyoka, N. M., Ren, J., Langmi, H. W., et al. Synthesis of rGO/Zr-MOF composite for hydrogen storage application. [J]Journal of Alloys and Compounds, 2017, 724: 450-455.
[10] U.S. Department of Energy. Hydrogen Storage Factsheet. Office of Energy Efficiency and Renewable Energy (EERE), 2020.
[11] Jain, I. P., Lal, C., Jain, A. Hydrogen storage in Mg: A most promising material. International Journal of Hydrogen Energy, 2010, 35(10), 5133–5144.
[12] Sandrock, G. A panoramic overview of hydrogen storage alloys from a gas reaction point of view. Journal of Alloys and Compounds, 1999, 293–295, 877–888.
[13] Li, Y., Yang, R. T. Hydrogen storage in metal–organic frameworks by bridged hydrogen spillover. Journal of the American Chemical Society, 2006, 128(25), 8136–8137.
[14] Thommes, M. et al. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution. Pure and Applied Chemistry, 2015, 87(9–10), 1051–1069.