What Is Chemisorption in Heterogeneous Catalysis?
Chemisorption—the formation of chemical bonds between gas-phase molecules and surface atoms—is the foundational step in heterogeneous catalysis. On supported metal catalysts, this process occurs on small metal crystallites, nanoparticles, and single atoms anchored to high surface area oxide materials. These chemisorbed species react with adjacent adsorbed molecules or gas-phase reactants to generate catalytic products.
Figure 1 shows a schematic diagram of the chemisorption step in a typical CO2 hydrogenation reaction with Pt/TiO2 catalyst.(1,2) Pt surface sites act as anchors for CO2 chemisorption until the species react with H2 and desorb.
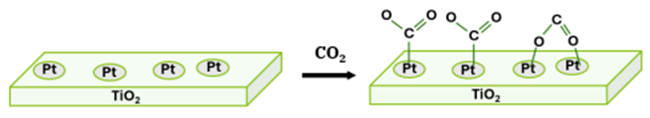
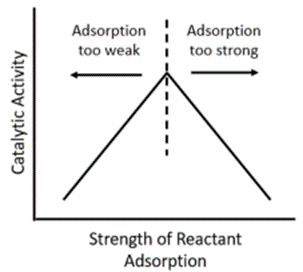
The chemisorption behavior of a catalyst directly impacts both reaction rate and selectivity toward desired products. Understanding and quantifying chemisorption is essential for catalyst design and performance optimization. Optimal catalytic performance requires a balance between the strength and quantity of chemisorbed species:
Binding Strength:
Too strong — hinders product formation as molecules adhere too tightly.
Too weak — reactants desorb before reacting.
Moderate — yields the highest catalytic activity (volcano curve).
✓ Quantity of Sites:
o The number of chemisorbed species correlates to the number of surface sites available, which can be used to quantify surface chemical properties of the catalyst
Chemisorption Measurement Methods (Static, Pulse, TPD, TPR, TPO)
Quantitative assessment of chemisorption requires techniques that can evaluate both the quantity and strength of adsorption sites, while also describing qualitative chemical properties. The broad range of chemisorption methods is shown in Table 1.
| Method | Measurement Focus |
| Static/Volumetric Chemisorption | Equilibrium uptake of gas molecules (closed system) |
| Pulse Chemisorption | Uptake of calibrated gas pulses |
| Temperature programmed esorption (TPD) | Desorption behavior upon heating — provides both site count and adsorption strength |
| Temperature programmed reduction (TPR) | Hydrogen consumption under H2/inert gas flow – provides metal dispersion, metal-support interactions, metal oxidation states |
| Temperature programmed oxidation (TPO) | Desorption behavior of oxidized surface species upon heating – quantifies carbon deposits, oxidation states |
| Temperature programmed surface reaction (TPSR) | Probe molecules react on surface, product desorption detected upon heating – describes active sites, reaction mechanisms, and kinetics |
| Steady-state isotopic transient kinetic analysis (SSITKA) | Steady-state conditions achieved with unlabeled reactant gas, then switched to isotopically labeled gas. Desorbed isotopically labeled products detected by mass spectrometry – describes kinetics and reaction intermediates |
If you’re planning TPD, TPR, or TPO experiments, you can explore chemisorption analyzers to compare configurations.
How Chemisorption Experiments Work (Step-by-Step Procedure)
A standard chemisorption experiment involves:
✓ Sample Preparation:
o Catalyst treated to yield clean surface sites.
o Introduction of the chemisorbing gas (typically at ambient temperature). ✓ Gas Switching & Flushing:
o Replace chemisorbing gas with inert gas.
Visit our Website: www.ami-instruments.com
✓ Controlled Heating:
o Linear temperature ramp.
o Desorption of chemisorbed species occurs at characteristic temperatures. ✓ Detection:
o Quantify desorbed species using calibrated detectors.
o Calculate site quantity and evaluate adsorption strength.
Example:
H₂ chemisorption was used by Li et al. on Ni/SiO₂ catalysts for ammonia decomposition. TPR and TPD experiments revealed both the number of available Ni sites and the oxidation state of surface Ni species.(3)
Chemisorption Analyzers by AMI (AMI-300 & AMI-400 Platforms)

AMI Chemisorption Analyzers automate the entire process with precise flow control and gas switching, programmable temperature ramps, quantitative detection and data analysis, and fully customizable experiment parameters through user-friendly software. The flagship AMI-300 platform delivers reproducible, operator-independent measurements, and specialized models are available for advanced chemisorption experiments—helping researchers optimize catalysts and advance reaction engineering. The AMI-300 IR enables real-time catalyst analysis with Fourier transform infrared spectroscopy (FTIR). The AMI-300 HP is engineered for industrially relevant high-pressure conditions up to 100 bar, and the AMI-300 SSITKA is integrated with steady-state isotopic transient kinetic analysis capabilities, alongside traditional chemisorption experiments (TPD, TPO/R, TPSR).
| AMI-300 Series |
| Unique features | Fully automated, highly customizable |
| Functions | Pulse chemisorption, temperature-programmed reduction (TPR), temperature-programmed oxidation (TPO), temperature programmed desorption (TPD), temperature-programmed surface reaction (TPSR), flow BET surface area analysis, steady state isotopic transient kinetic analysis (SSITKA) |
| Sample loading | 0.1-5g |
| Temperature range | RT – 1200 oC with rapid cooling |
| Ramp rate | 0.1-50 oC/min |
| Operating pressure | Ambient – 100 bar (AMI-300 HP) |
| Thermocouples | Bed thermocouple, furnace thermocouple |
| Gas flow rates | 2-100 sccm |
| Reactor types | Quartz U-tubes (6mm, 8mm, 10mm) |
| Detector | TCD (sensitive tungsten-rhenium filament) |
| Mass flow controllers | 3 (4 optional) |
| Optional add-ons | FTIR (AMI-300 IR), vapor generator, mass spectrometer, FID, methanator reactor, harsh chemistry, SSITKA (AMI-300 SSITKA), custom sample holders |
The AMI-400 platform expands on the previous model with improved precision and industry leading safety features. In addition, the compact AMI-400 TPx offers the same automation capabilities with outstanding economic efficiency and space-saving design.
| AMI-400 Series |
| Unique features | Fully automated smart gas interface; integrated safety features including exhaust fan, alarm system, and self-locking door; high precision thermocouples and MFCs |
| Experiments | Pulse chemisorption, temperature-programmed reduction (TPR), temperature-programmed oxidation (TPO), temperature-programmed desorption (TPD), temperature-programmed surface reaction (TPSR), flow BET surface area analysis, |
| Sample loading | 0.1-5g |
| Temperature range | RT – 1200 oC, -130 oC – 1100 oC (optional) |
| Ramp rate | 0.1-50 oC/min |
| Operating pressure | Ambient |
| Thermocouples | Bed thermocouple, furnace thermocouple, overtemperature protection thermocouple |
| Gas flow rates | 0-100 sccm (+/- 1% accuracy) |
| Reactor types | Quartz U-tubes (6mm, 8mm, 10mm) |
| Detector | TCD (sensitive tungsten-rhenium filament) |
| Mass flow controllers | 1 (2-4 optional) |
| Optional add-ons | Vapor generator, mass spectrometer, FID, FTIR, methanator reactor |
Conclusion: Why Chemisorption Data Improves Catalyst Performance
Chemisorption is an integral reaction step in heterogeneous catalysis, and it can be employed as a powerful tool for detailed catalyst analysis. Through advanced, automated tools like the AMI Chemisorption Series, scientists can describe the elemental, surface-level interactions driving catalyst performance and use that understanding to implement efficient catalyst design.
If you’re selecting a system for catalyst characterization, Explore AMI’s chemisorption analyzers—or call our team to get a recommendation for your catalyst workflow.
- References
- Hu, X.; Xu, D.; Jiang, J. Strong metal-support interaction… Angew. Chem. Int. Ed. 2025, 64, e202419103.
- Su, G.-X.; Wu, M.-Y.; Wang, W.-W.; Jia, C.-J. Pt nanoparticles… ACS Appl. Nano Mater. 2025, 8, 9164–6176.
- Li, S.; Liu, X.; Guo, Y.; Wang, Y. Highly active and stable Ni@SiO2… Fuel 2024, 368, 131543.





